| CPC C07D 213/30 (2013.01) [A61K 47/30 (2013.01); C07C 237/22 (2013.01); C07D 209/42 (2013.01); C07D 231/56 (2013.01); C07D 235/10 (2013.01); C07D 235/14 (2013.01); C07D 235/30 (2013.01); A61K 9/0043 (2013.01)] | 9 Claims |
|
1. A pharmaceutical composition comprising a compound or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, wherein the compound is of Formula (II):
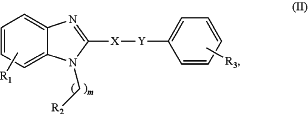 wherein R1 is selected from hydrogen; halogen; nitro; cyano; amino; hydroxy; carboxy; (C1-C6)alky; halo(C1-C6)alkyl; (C1-C6)alkoxy; halo(C1-C6)alkoxy; (C3-C10)cycloalkyl; halo(C3-C10)cycloalkyl; (C3-C10)cycloalkylthio; halo(C3-C10)cycloalkythio; (C3-C10)heterocycloalkyl; halo(C3-C10)heterocycloalky; (C1-C6)alkylamino; di(C1-C6)alkylamino; (C1-C6)alkylthio; halo(C1-C6)alkylthio; (C1-C6)alkylsulfinyl; halo(C1-C6)alkylsulfinyl; (C1-C6)alkylsulfonyl; halo(C1-C6)alkylsulfonyl; (C3-C10)cycloalkylsulfinyl; halo(C3-C10)cycloalkylsulfinyl; (C3-C10)cycloalkylsulfonyl; halo(C3-C10)cycloalkylsulfonyl; aryl, heteroaryl, arylamine, or heterocyclyl, optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, or halo(C1-C6)alkoxy; and an aryl ring fused to the relevant phenyl ring, either ring being optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, halo(C1-C6)alkoxy or arylamine;
R3 is selected from halogen; nitro; cyano; amino; hydroxy; carboxy; (C1-C6)alkyl; halo(C1-C6)alkyl; (C1-C6)alkoxy; halo(C1-C6)alkoxy; (C3-C10)cycloalkyl; halo(C3-C10)cycloalkyl; (C3-C10)cycloalkylthio; halo(C3-C10)cycloalkylthio; (C3-C10)heterocycloalkyl; halo(C3-C10)heterocycloalkyl; (C1-C6)alkylamino; di(C1-C6)alkylamino; (C1-C6)alkylthio; halo(C1-C6)alkylthio; (C1-C6)alkylsulfinyl; halo(C1-C6)alkylsulfinyl; (C1-C6)alkylsulfonyl; halo(C1-C6)alkylsulfonyl; (C3-C10)cycloalkylsulfinyl; halo(C3-C10)cycloalkylsulfinyl; (C3-C10)cycloalkylsulfonyl; halo(C3-C10)cycloalkylsulfonyl; aryl, heteroaryl, arylamine, or heterocyclyl, optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, or halo(C1-C6)alkoxy; and an aryl ring fused to the relevant phenyl ring, either ring being optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, halo(C1-C6)alkoxy or arylamine;
X—Y is CH═CH, C≡C, or N═N;
R2 is selected from (C1-C6)alkyl; halo(C1-C6)alkyl; (C2-C6)alkenyl; halo(C2-C6)alkenyl;
(C2-C6)alkynyl; halo(C2-C6)alkynyl; (C3-C10)cycloalkyl; halo(C3-C10)cycloalkyl; heterocyclyl, aryl, or heteroaryl, each optionally substituted with one or more groups independently selected from halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, or halo(C1-C6)alkoxy; and
m is 0 to 4.
|
|
7. A compound of Formula (II):
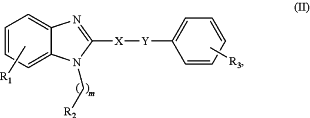 or a pharmaceutically acceptable salt thereof,
wherein R1 is selected from hydrogen; halogen; nitro; cyano; amino; hydroxy; carboxy; (C1-C6)alky; halo(C1-C6)alkyl; (C1-C6)alkoxy; halo(C1-C6)alkoxy; (C3-C10)cycloalkyl; halo(C3-C10)cycloalkyl; (C3-C10)cycloalkylthio; halo(C3-C10)cycloalkythio; (C3-C10)heterocycloalkyl; halo(C3-C10)heterocycloalky; (C1-C6)alkylamino; di(C1-C6)alkylamino; (C1-C6)alkylthio; halo(C1-C6)alkylthio; (C1-C6)alkylsulfinyl; halo(C1-C6)alkylsulfinyl; (C1-C6)alkylsulfonyl; halo(C1-C6)alkylsulfonyl; (C3-C10)cycloalkylsulfinyl; halo(C3-C10)cycloalkylsulfinyl; (C3-C10)cycloalkylsulfonyl; halo(C3-C10)cycloalkylsulfonyl; aryl, heteroaryl, arylamine, or heterocyclyl, optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, or halo(C1-C6)alkoxy; and an aryl ring fused to the relevant phenyl ring, either ring being optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, halo(C1-C6)alkoxy or arylamine;
R3 is selected from halogen; nitro; cyano; amino; hydroxy; carboxy; (C1-C6)alkyl; halo(C1-C6)alkyl; (C1-C6)alkoxy; halo(C1-C6)alkoxy; (C3-C10)cycloalkyl; halo(C3-C10)cycloalkyl; (C3-C10)cycloalkylthio; halo(C3-C10)cycloalkylthio; (C3-C10)heterocycloalkyl; halo(C3-C10)heterocycloalkyl; (C1-C6)alkylamino; di(C1-C6)alkylamino; (C1-C6)alkylthio; halo(C1-C6)alkylthio; (C1-C6)alkylsulfinyl; halo(C1-C6)alkylsulfinyl; (C1-C6)alkylsulfonyl; halo(C1-C6)alkylsulfonyl; (C3-C10)cycloalkylsulfinyl; halo(C3-C10)cycloalkylsulfinyl; (C3-C10)cycloalkylsulfonyl; halo(C3-C10)cycloalkylsulfonyl; aryl, heteroaryl, arylamine, or heterocyclyl, optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, or halo(C1-C6)alkoxy; and an aryl ring fused to the relevant phenyl ring, either ring being optionally substituted with halogen, nitro, cyano, amino, hydroxy, carboxy, (C1-C6)alkyl, halo(C1-C6)alkyl, (C1-C6)alkoxy, halo(C1-C6)alkoxy or arylamine;
X—Y is C≡C;
R2 is a (C2-C6)alkenyl or a (C2-C6)alkynyl; and
m is 1.
|