| CPC C07C 59/54 (2013.01) [C07C 35/50 (2013.01); C07C 39/23 (2013.01); C07C 43/23 (2013.01); C07C 49/683 (2013.01); C07C 49/83 (2013.01); C07C 215/70 (2013.01); C07C 255/47 (2013.01); C07C 271/34 (2013.01); C07C 307/02 (2013.01); C07C 309/66 (2013.01); C07D 249/04 (2013.01); C07F 9/091 (2013.01); C07F 9/572 (2013.01); C07C 2602/22 (2017.05)] | 6 Claims |
|
1. A method of treating inflammatory bowel diseases (IBD) comprising administering to a subject in need thereof an effective amount of a compound having the following formula:
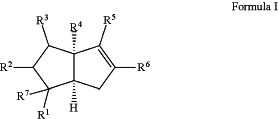 prodrugs or salts thereof wherein,
R1 is alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R1 is optionally substituted with one or more, the same or different, R10;
R2 is hydrogen, alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R2 is optionally substituted with one or more, the same or different, R10;
R3 is hydrogen, alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R3 is optionally substituted with one or more, the same or different, R10;
R4 is alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R4 is optionally substituted with one or more, the same or different, R10;
R5 is alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R5 is optionally substituted with one or more, the same or different, R10;
R6 is lipid or alkyl, wherein R6 is optionally terminally substituted with a hydroxy, carboxy, or phosphate, wherein the hydroxy, carboxy, or phosphate are optionally further substituted with R10;
R7 is hydrogen, alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R7 is optionally substituted with one or more, the same or different, R10; or
R1 and R7 together are an oxo or oxime, wherein the oxime is optionally substituted with one or more, the same or different, R10;
R10 is alkyl, halogen, nitro, cyano, hydroxy, amino, mercapto, formyl, carboxy, carbamoyl, alkoxy, hydroxyalkyl, alkylthio, thioalkyl, alkylamino, aminoalkyl, (alkyl)2amino, alkanoyl, alkoxycarbonyl, alkylsulfinyl, alkylsulfonyl, arylsulfonyl, aminosulfonyl, carbocyclyl, benzoyl, benzyl, aryl, or heterocyclyl, wherein R10 is optionally substituted with one or more, the same or different, R11; and
R11 is halogen, nitro, cyano, hydroxy, trifluoromethoxy, trifluoromethyl, amino, formyl, carboxy, carbamoyl, mercapto, sulfamoyl, methyl, ethyl, methoxy, ethoxy, isopropoxy, tert-butoxy, hydoxymethyl, hydroxyethyl, thiomethyl, thioethyl, aminomethyl, aminoethyl, acetyl, acetoxy, methylamino, ethylamino, dimethylamino, diethylamino, N-methyl-N-ethylamino, acetylamino, N-methylcarbamoyl, N-ethylcarbamoyl, N,N-dimethylcarbamoyl, N,N-diethylcarbamoyl, N-methyl-N-ethylcarbamoyl, methylthio, ethylthio, methylsulfinyl, ethylsulfinyl, mesyl, ethylsulfonyl, methoxycarbonyl, ethoxycarbonyl, isopropoxycarbonyl, tert-butoxycarbonyl, N-methylsulfamoyl, N-ethylsulfamoyl, N,N-dimethylsulfamoyl, N,N-diethylsulfamoyl, N-methyl-N-ethylsulfamoyl, benzoyl, benzyl, carbocyclyl, aryl, or heterocyclyl.
|