| CPC A61M 16/0666 (2013.01) [A61M 16/0066 (2013.01); A61M 16/0622 (2014.02); A61M 16/0683 (2013.01); A61M 16/0875 (2013.01); A61M 16/0616 (2014.02); A61M 16/1055 (2013.01); A61M 16/107 (2014.02); A61M 2205/0216 (2013.01); A61M 2207/00 (2013.01)] | 1 Claim |
|
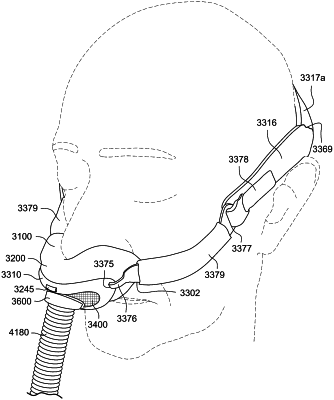
1. A patient interface for delivering pressurized gas to a patient at a therapeutic pressure of between 4 cmH2O and 30 cmH2O, the patient interface comprising:
a frame including a connection port sized and structured to receive a flow of air at the therapeutic pressure,
a plenum chamber pressurisable to the therapeutic pressure and removably connected to the frame;
a seal-forming structure constructed and arranged to seal against the patient's face around the patient's nares such that the flow of air at the therapeutic pressure is delivered to the patient's nares, the seal-forming structure constructed and arranged to maintain the therapeutic pressure in the plenum chamber throughout the patient's respiratory cycle in use, the seal-forming structure having a pair of naris ports and a nasal sling positioned between the naris ports, each of the pair of naris ports corresponding to one of the patient's nares to provide pressurized gas to the corresponding naris, and the nasal sling being structured and positioned to be located adjacent to the patient's columella and prevent the patient's nose from extending through the naris ports;
a vent structure formed on the frame and configured to allow a continuous flow of gases exhaled by the patient to pass from within the plenum chamber to ambient;
a positioning and stabilising structure configured to hold the seal-forming structure in a therapeutically effective position on the patient's face, the positioning and stabilising structure having a pair of rigidiser arms connected to the frame and a strap removably connected to the pair of rigidiser arms, the strap being length-extensible, and the strap being constructed and arranged so that at least a portion overlies a lateral region of the patient's head superior to an otobasion superior of the patient's head in use;
a retaining structure integrally moulded to the plenum chamber and configured to removably connect the plenum chamber to the frame; and
a gas delivery tube connected to the frame at the connection port, the gas delivery tube configured to direct the flow of air at the therapeutic pressure to the plenum chamber;
wherein the patient interface is configured to leave the patient's mouth uncovered;
wherein the seal-forming structure and the plenum chamber are moulded from a first silicone material and the retaining structure is moulded from a second silicone material with a higher durometer than the first silicone material such that the retaining structure is more rigid than the seal-forming structure and the plenum chamber; and
wherein the plenum chamber further comprises a sealing lip configured to engage the frame such that an increase in air pressure within the plenum chamber causes a sealing force between the sealing lip and the frame to increase.
|