| CPC A61L 27/3804 (2013.01) [A61L 27/3604 (2013.01); A61L 27/3687 (2013.01); A61L 27/3691 (2013.01); A61L 27/52 (2013.01); C12N 5/0609 (2013.01); A61L 2400/06 (2013.01); A61L 2430/40 (2013.01); C12N 2500/84 (2013.01)] | 3 Claims |
|
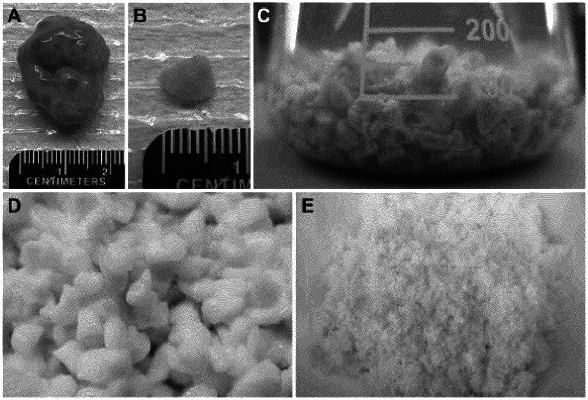
1. A lyophilisate comprising a decellularized ovarian tissue, wherein the lyophilisate comprises an ovarian-derived extracellular matrix in a concentration between 1 mg/ml to 10 mg/ml, at least one biocompatible crosslinking reagent selected from the group consisting of lysyl oxidase, genipin, ribose, rose bengal, and combinations thereof, wherein the lyophilisate includes adhesion sites for a follicle.
|