| CPC A61K 9/4808 (2013.01) [A61B 5/14503 (2013.01); A61B 5/14539 (2013.01); A61B 10/02 (2013.01); A61J 3/07 (2013.01); A61K 9/0002 (2013.01); A61K 9/0012 (2013.01); A61K 9/0021 (2013.01); A61K 9/0065 (2013.01); A61K 9/0092 (2013.01); A61K 9/4866 (2013.01); A61K 38/28 (2013.01); A61M 5/14276 (2013.01); A61M 5/1454 (2013.01); A61M 5/158 (2013.01); A61M 5/2033 (2013.01); A61M 5/329 (2013.01); A61M 5/3295 (2013.01); A61M 31/002 (2013.01); A61M 37/0015 (2013.01); A61M 37/0069 (2013.01); A61N 1/325 (2013.01); A61N 1/36007 (2013.01); A61B 2010/0208 (2013.01); A61B 10/0233 (2013.01); A61B 10/06 (2013.01); A61M 2005/14284 (2013.01); A61M 2005/1581 (2013.01); A61M 2005/1585 (2013.01); A61M 2037/0023 (2013.01); A61M 2037/0053 (2013.01); A61M 2205/0238 (2013.01); A61M 2205/21 (2013.01); A61M 2210/1053 (2013.01); A61M 2210/106 (2013.01); A61N 1/0509 (2013.01)] | 26 Claims |
|
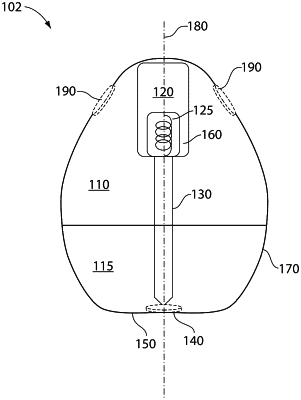
1. A self-righting article configured for administration to a subject, the self-righting article comprising:
a tissue interfacing component comprising a solid therapeutic agent, the solid therapeutic agent present in the tissue interfacing component in an amount of greater than or equal to 10 weight percentage of the total weight of the tissue interfacing component,
wherein the self-righting article is a monostatic body due to a center of mass of the self-righting article and/or the shape of the self-righting article, such that the self-righting article has a single stable resting position.
|