| CPC A61K 31/5377 (2013.01) [A61P 37/00 (2018.01); C12Q 1/6883 (2013.01); C12Q 2600/158 (2013.01)] | 18 Claims |
|
1. A method of determining a dose of a treatment compound for treating a subject having systemic lupus erythematosus (SLE), comprising:
(a) measuring the gene expression level of IKZF3 in a sample from the subject; and
(b) determining the dose of the treatment compound to be 0.45 mg or higher per day if a score calculated based on the gene expression level of IKZF3 in the sample is higher than a reference level,
wherein the treatment compound is a compound of Formula I:
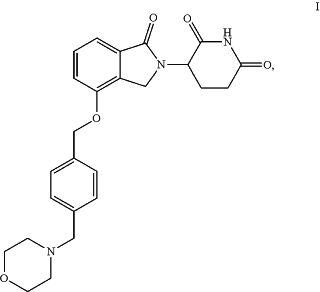 or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, tautomer or racemic mixture thereof.
|