| CPC A61K 31/519 (2013.01) [A61K 9/2027 (2013.01); A61K 9/2054 (2013.01); A61K 9/2077 (2013.01); A61K 9/284 (2013.01); A61K 31/496 (2013.01)] | 6 Claims |
|
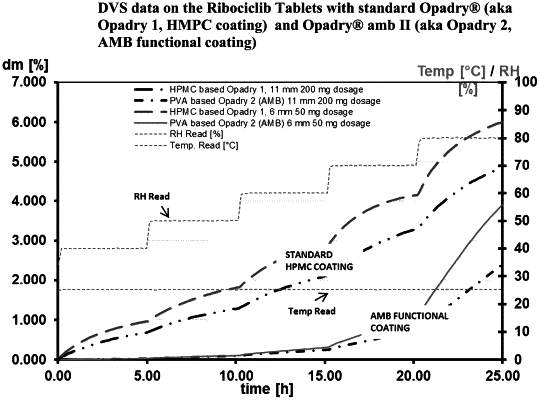
1. A coated pharmaceutical oral tablet comprising ribociclib succinate, the coated pharmaceutical oral tablet comprising a tablet core and a coating, wherein the % of ribociclib succinate (w/w) is at least 50% of the tablet core, and the coating is a polyvinyl alcohol (PVA)-based advanced moisture barrier coating.
|