| CPC A61K 31/198 (2013.01) [A61P 11/00 (2018.01); A61P 31/14 (2018.01)] | 20 Claims |
|
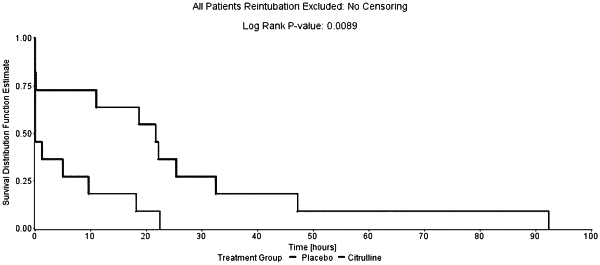
1. A method for treating endothelial dysfunction in a patient, the method comprising administering an effective amount of citrulline to the patient, wherein the effective amount of citrulline is an amount sufficient to reduce the uncoupling of endothelial nitric oxide synthase (eNOS) and/or an amount sufficient to reduce the formation of free radicals;
wherein the patient experiencing endothelial dysfunction has plasma levels of D-dimer above 250 ng/mL; and/or
wherein the effective amount of citrulline is sufficient to reduce plasma level of angiopoietin-2 by a detectable amount.
|