| CPC A61F 2/30771 (2013.01) [A61F 2/3859 (2013.01); A61F 2/389 (2013.01); A61F 2/4081 (2013.01); A61F 2/4202 (2013.01); A61F 2/4261 (2013.01); A61F 2/442 (2013.01); A61L 27/06 (2013.01); A61L 27/18 (2013.01); A61F 2/2803 (2013.01); A61F 2002/30069 (2013.01); A61F 2002/30224 (2013.01); A61F 2002/30326 (2013.01); A61F 2002/30383 (2013.01); A61F 2002/30896 (2013.01); A61F 2002/3093 (2013.01); A61F 2002/3863 (2013.01); A61F 2002/4205 (2013.01); A61F 2002/4207 (2013.01); A61F 2310/00017 (2013.01); A61F 2310/00023 (2013.01); A61F 2310/00029 (2013.01); A61F 2310/00293 (2013.01); A61F 2310/00359 (2013.01); A61L 2430/02 (2013.01); A61L 2430/38 (2013.01)] | 10 Claims |
|
1. A glenoid implant for a shoulder, the glenoid implant comprising:
(a) a bulk glenoid implant;
(b) a planar face defining an exterior bone-engaging surface of the bulk glenoid implant, the exterior bone-engaging surface sized and configured to contact a resected surface of a glenoid;
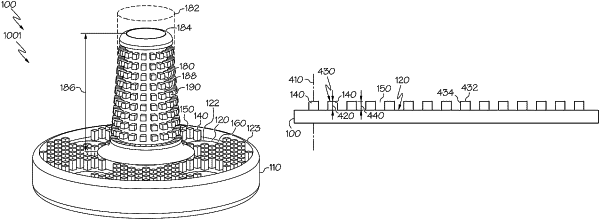
(c) pillars sized and configured for contacting a hard tissue, the pillars being distributed on the exterior bone-engaging surface, across an area of at least 30 mm2, and extending distally and perpendicularly from the planar face, and each pillar (i) being integral to the bulk glenoid implant, (ii) having a distal end, (iii) having a transverse area of (100×100) μm2 to (10,000×10,000) μm2, and (iv) having a height of 200 μm to 2,500 μm;
(d) slots to be occupied by the hard tissue, the slots being defined by the pillars and each slot having a width of 100 μm to 10,000 μm as measured along a shortest distance between adjacent pillars; and
(e) at least one support member sized and configured for contacting the hard tissue, the at least one support member being positioned on the exterior bone-engaging surface among the pillars, extending distally from the exterior bone-engaging surface, having a transverse area greater than the transverse area of any of the pillars, having a height greater than the height of any of the pillars, and having at least one region lacking support member pillars and at least one region comprising support member pillars;
wherein the glenoid implant has a Young's modulus of elasticity of at least 3 GPa, and has a ratio of (i) the sum of the volumes of the slots to (ii) a sum of the volumes of the pillars and the volumes of the slots of 0.40:1 to 0.90:1
wherein:
one or more pillars have dimensions that differ from those of other pillars, such that the transverse areas and/or heights, and thus volumes, of the one or more pillars differ from those of the other pillars; and
the one or more pillars are distributed peripherally with respect to the exterior bone-engaging surface and are intended for insertion into cortical bone, and the other pillars are distributed centrally with respect to the exterior bone-engaging surface and are intended for insertion into cancellous bone; and
wherein the pillars are non-porous.
|