| CPC A01N 43/40 (2013.01) [A01P 1/00 (2021.08); C07D 405/12 (2013.01)] | 1 Claim |
|
1. A method of preparing 4-(N-methyl) aminopiperidine myricetin derivatives containing sulfonamide, the synthetic route of which is as follows: mixing myricetin with potassium carbonate (K2CO3) and N,N-dimethylformamide (DMF) and adding with methyl iodide (CH3I) to perform reaction, and followed by adding absolute ethyl alcohol (CH3CH2OH) to heat to reflux to obtain a clarified solution and adding concentrated hydrochloric acid (HCl) into the clarified solution under reflux, thereby
(1) obtaining 3-Hydroxy-3′,4′,5′,5,7-pentamethoxy myricetin (intermediate a)
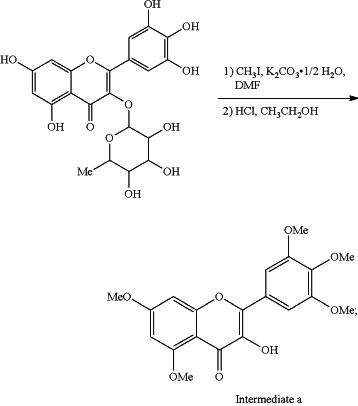 (2) combining the intermediate a with dibromoalkanes (Br(CH2)nBr) and potassium carbonate as a catalyst and DMF as a solvent for 1 day (d) at room temperature (rt) to obtain 3-bromo-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromene-4-one (intermediate b):
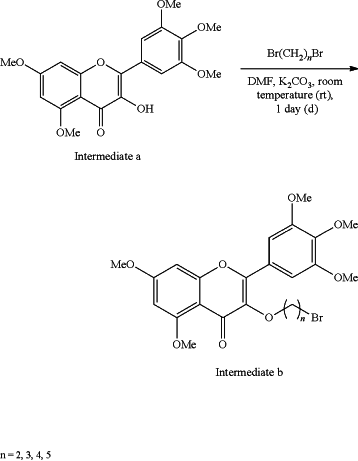 (3) mixing the intermediate b and 4-(N-methyl) amino-N-Boc piperidine by taking potassium carbonate as a catalyst and acetonitrile as a solvent to prepare 3-(4-(N-methyl) amino-N-Boc piperidine)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromene-4-one (intermediate c) under reflux and stirring at 80° C., wherein the Boc of intermediate c is a tert-butylcarbonyl protecting group:
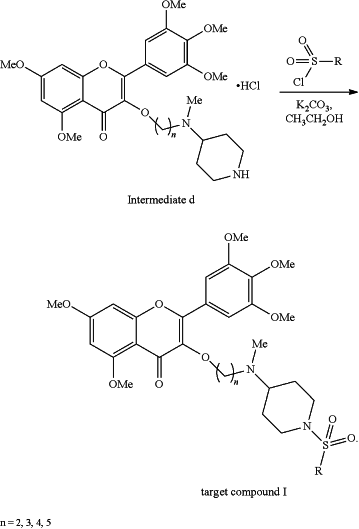
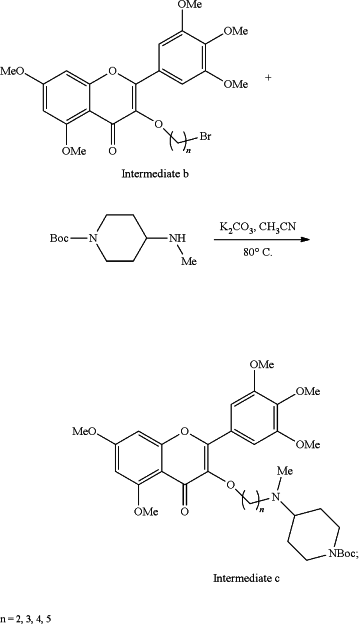 (4) removing the tert-butylcarbonyl (Boc) protecting group from the intermediate c with methanol (CH3OH) as a solvent and hydrochloride (HCl) at room temp (rt) for 2 hours (2h) to obtain hydrochloride (intermediate d) of the 3-(4-(N-methyl) aminopiperidine)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromene-4-one
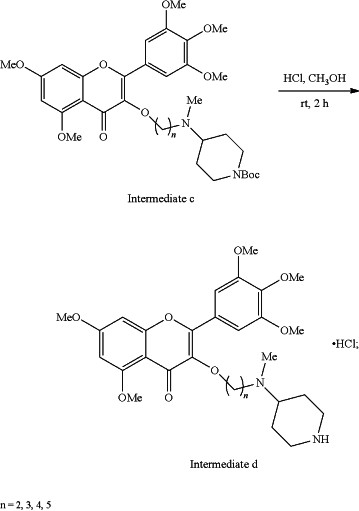 (5) mixing the intermediate d and substituted sulfonyl chloride by taking potassium carbonate as a catalyst and absolute ethyl alcohol as a solvent to prepare the 4-(N-methyl) aminopiperidine myricetin derivatives containing sulfonamides (target compound I)
 |