| CPC A01N 37/42 (2013.01) [A01P 21/00 (2021.08); C07C 59/90 (2013.01); C07C 69/738 (2013.01)] | 22 Claims |
|
1. A compound of Formula (I) or an enantiomer, salt, and/or solvate thereof:
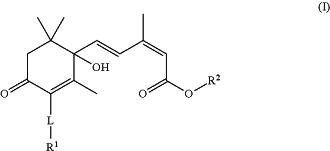 wherein
L is —C═C— or —C≡C—;
R1 is C1-10alkyl, C2-10alkenyl, C2-10alkynyl, (CH2)0-2C3-10cycloalkyl, (CH2)0-2aryl, (CH2)0-2heterocycloalkyl, or (CH2)0-2heteroaryl, each being optionally substituted with one or more of halo, CN, OH, NH2, C1-10alkyl, C2-10alkenyl, C2-10alkynyl, NH(C1-6alkyl), N(C1-6 alkyl)(C1-6alkyl), OC1-6alkyl, OC2-6alkenyl, OC2-6alkynyl, (CH2)0-2C3-10cycloalkyl, (CH2)0-2aryl, (CH2)0-2heterocycloalkyl, (CH2)0-2heteroaryl, O(CH2)0-2C3-10cycloalkyl, O(CH2)0-2aryl, O(CH2)0-2heterocycloalkyl, or O(CH2)0-2heteroaryl, the latter 16 groups being optionally substituted with one or more of halo, OH, NH2, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, OC1-16alkyl, OC2-6alkenyl, or OC2-6alkynyl; and
R2 is H, C1-10alkyl, C2-10alkenyl, C2-10alkynyl, cycloalkyl, aryl, heterocycloalkyl or heteroaryl, the latter 7 groups being optionally substituted with one or more of halo, OH, NH2, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, OC1-16alkyl, OC2-6alkenyl, or OC2-6alkynyl,
wherein each alkyl, alkenyl, and alkynyl are optionally fluorosubstituted.
|