| CPC C07K 5/06026 (2013.01) [C07D 235/10 (2013.01); C07D 235/14 (2013.01); C07K 5/02 (2013.01); A61K 38/00 (2013.01)] | 15 Claims |
|
1. A method of treating bacterial infections, parasite infections, fungal infections, cancer, autoimmune disorders, neurodegenerative diseases and disorders, inflammatory disorders, or muscular dystrophy, in a subject or for achieving immunosuppression in transplanted organs or tissues in a subject, said method comprising:
administering to the subject in need thereof a compound of Formula (I):
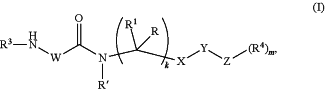 wherein
R is H or C1-6 alkyl;
R′ is H or C1-6 alkyl;
R1 is H or C1-6 alkyl;
or R and R1 are taken together with the carbon to which they are attached to form a C3-8 cycloalkyl ring;
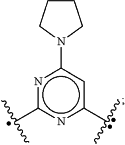
R2 is independently selected at each occurrence thereof from
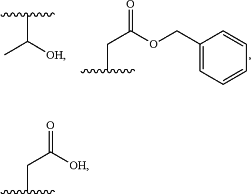 or —(CH2)nC(O)NR6R7;
R3 is independently selected at each occurrence thereof from the group consisting of H, C1-12 alkyl, -Boc, —C(O)(CH2)nR5, —(CH2)nC(O)R5, —C(O)OR5, —C(O)(CH2)nNR6R7, —S(O)2R5, monocyclic and bicyclic heteroaryl, monocyclic and bicyclic heterocyclyl, and monocyclic and bicyclic non-aromatic heterocycle, wherein monocyclic and bicyclic heteroaryl, monocyclic and bicyclic heterocyclyl, and monocyclic and bicyclic non-aromatic heterocycle can be optionally substituted from 1 to 3 times with R8;
R4 is H, halogen, NH2, NHCOOC1-12 alkyl, or C1-12 alkyl;
R5 is selected from the group consisting of C1-12 alkyl, monocyclic or bicyclic C3-10 cycloalkyl, C3-12 cycloalkylalkyl, C1-12 alkoxy, monocyclic and bicyclic aryl, monocyclic and bicyclic heteroaryl, monocyclic and bicyclic heterocyclyl, and monocyclic and bicyclic non-aromatic heterocycle, wherein C1-12 alkyl, monocyclic or bicyclic C3-10 cycloalkyl, C3-12 cycloalkylalkyl, C1-12 alkoxy, monocyclic and bicyclic aryl, monocyclic and bicyclic heteroaryl, monocyclic and bicyclic heterocyclyl, and monocyclic and bicyclic non-aromatic heterocycle can be optionally substituted from 1 to 3 times with R8;
R6 and R7 are each independently selected from the group consisting of H, C1-6 alkyl, and arylalkyl;
or R6 and R7 are taken together with the nitrogen to which they are attached to form a piperidine, pyrrolidine, azepane, or morpholine ring, wherein piperidine, pyrrolidine, azepane, or morpholine ring can be optionally substituted 1 to 3 times with R9;
R8 is selected independently at each occurrence thereof from the group consisting of halogen, C1-6 alkyl, C3-8 cycloalkyl, aryl, and arylalkyl, wherein C1-6 alkyl, C3-8 cycloalkyl, aryl, and arylalkyl can be optionally substituted 1 to 3 times with R9;
R9 is selected from the group consisting of H, halogen, C1-6 alkyl, C3-8 cycloalkyl, and aryl, wherein C1-6 alkyl can be optionally substituted 1 to 3 times with halogen;
W is CHR2, NR2, or
 X is selected from the group consisting of —C(O)—NH—, monocyclic and bicyclic heteroaryl, monocyclic and bicyclic heterocyclyl, and monocyclic and bicyclic non-aromatic heterocycle;
Y is optional and, if present, is —(CH2)m—;
Z is optional and, if present, is aryl or bicyclic heteroaryl;
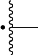 is the point of attachment to NHR3 moiety;
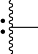 is the point of attachment to C(O) moiety;
k is 1 or 2;
m is 0, 1, or 2; and
n is 0, 1, 2, 3, or 4,
with the proviso that R2 is not —CH2C(O)NH2, —CH2C(O)NHCH2C(CH3)3, or —(CH2)2C(O)NH2,
a pharmaceutically acceptable salt thereof or a solvate thereof,
wherein the autoimmune disorder is selected from the group consisting of arthritis, colitis, multiple sclerosis, lupus, Sjogren Syndrome, Systemic Lupus Erythematosus, lupus nephritis, glomerulonephritis, Rheumatoid Arthritis, Inflammatory bowel disease (IBD), ulcerative colitis, Crohn's diseases, Psoriasis, and asthma;
the inflammatory disorder is Crohn's disease;
the cancer is selected from the group consisting of neoplastic disorders, hematologic malignances, lymphocytic malignancies, mantel cell lymphoma, leukemia, Waldenstrom Macroglobulinemia, pancreatic cancer, bladder cancer, colorectal cancer, breast cancer, metastatic breast cancer, prostate cancer, androgen-dependent and androgen-independent prostate cancer, renal cancer, metastatic renal cell carcinoma, hepatocellular cancer, lung cancer, non-small cell lung cancer (NSCLC), bronchioloalveolar carcinoma (BAC), adenocarcinoma of the lung, ovarian cancer, progressive epithelial or primary peritoneal cancer, cervical cancer, gastric cancer, esophageal cancer, head and neck cancer, squamous cell carcinoma of the head and neck, melanoma, neuroendocrine cancer, metastatic neuroendocrine tumors, brain tumors, glioma, anaplastic oligodendroglioma, adult glioblastoma multiforme, and adult anaplastic astrocytoma, bone cancer, and soft tissue sarcoma; and
the neurodegenerative disease or disorder is Amyotrophic Lateral Sclerosis (ALS) or Multiple Sclerosis (MS).
|