| CPC C07D 491/107 (2013.01) [A61P 25/00 (2018.01); A61P 25/02 (2018.01); A61P 25/04 (2018.01); A61P 29/00 (2018.01); C07D 405/14 (2013.01)] | 20 Claims |
|
1. A method for treating a neurologic disease selected from inflammatory and neuropathic pain, comprising administering to a human patient an effective amount of a pharmaceutical formulation containing a compound of Formula I to treat the neurologic disease, wherein Formula I is represented by:
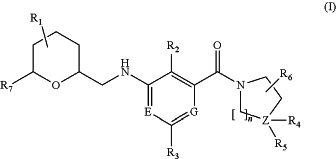 or a salt thereof; wherein:
R1 is a group selected from among —H, -halogen, —CN, —O—C1-C4-alkyl, —C1-C4-alkyl, —CH═CH2, —C≡CH, —CF3, —OCF3, —OCF2H, and —OCFH2;
R7 is a ring selected from among —C3-C5-cycloalkyl, —C5-C10-aryl, and —C5-C10-heteroaryl,
wherein the ring R7 is optionally substituted with one or more groups selected from among —CF3, —O—CF3, —S—CF3, —CN, —C1-C6-alkyl, —C(CH3)2—CN, and -halogen, or wherein the ring R7 is optionally substituted with one or more groups selected from among —O—C1-C6-alkyl and —C3-C8-cycloalkyl;
R2 is selected from among —H, -halogen, —CN, —O—C2-C4-alkyl, —C1-C4-alkyl, —CH═CH2, —C≡CH, —CF3, —OCF3, —OCF2H, and —OCFH2;
R3 is selected from among —H, -methyl, -ethyl, -propyl, -i-propyl, -cyclopropyl, —OCH3, —CF3, and —CN;
n is 1, 2, or 3;
G and E are N;
Z is C;
R4 denotes —H, and R5 is -L1-R18, wherein L1 is selected from among —NH—, —N(C1-C4-alkyl)-, and a bond, and R18 is —C3-C8-cycloalkyl or —C3-C8-heterocyclyl, wherein R18 is optionally substituted by one or more groups selected from among halogen, —CF3, —OCF3, —CN, —OH, —O—C1-C4-alkyl, —C1-C6-alkyl, —NH—C(O)—C1-C6-alkyl, —N(C1-C4-alkyl)-C(O)—C1-C6-alkyl, and —C(O)—C1-C6-alkyl; and
R6 is selected from among —H, —C1-C4-alkyl, —OH, —O—C1-C4-alkyl, -halogen, —CN, —CF3, and —OCF3.
|