| CPC A61K 31/497 (2013.01) [A61K 31/7034 (2013.01); A61P 13/12 (2018.01)] | 21 Claims |
|
1. A method of treating chronic kidney disease in a human patient, the method comprising administering to the patient in need thereof, a combination of zibotentan, N-(3-methoxy-5-methylpyrazin-2-yl)-2-[4-(1,3,4-oxadiazol-2-yl)phenyl]pyridine-3-sulfonamide,
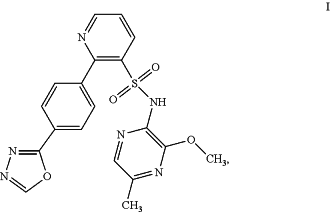 or a pharmaceutically acceptable salt thereof, and dapagliflozin.
|