| CPC A61B 6/504 (2013.01) [A61B 5/0066 (2013.01); A61B 5/0075 (2013.01); A61B 5/055 (2013.01); A61B 5/7267 (2013.01); A61B 5/742 (2013.01); A61B 5/7475 (2013.01); A61B 6/032 (2013.01); A61B 6/037 (2013.01); A61B 6/463 (2013.01); A61B 6/467 (2013.01); A61B 6/481 (2013.01); A61B 6/5205 (2013.01); A61B 8/12 (2013.01); A61B 8/14 (2013.01); A61K 49/04 (2013.01); G06F 18/10 (2023.01); G06T 7/0012 (2013.01); G06V 10/20 (2022.01); G06V 10/245 (2022.01); G06V 10/761 (2022.01); G06V 10/764 (2022.01); G06V 10/82 (2022.01); G06T 2207/10081 (2013.01); G06T 2207/10088 (2013.01); G06T 2207/10101 (2013.01); G06T 2207/10132 (2013.01); G06T 2207/20081 (2013.01); G06T 2207/30048 (2013.01); G06T 2207/30101 (2013.01); G06V 10/247 (2022.01)] | 30 Claims |
|
1. A system for tracking post-treatment progression of coronary artery disease (CAD) based on medical image analysis of a medical image of a subject to facilitate determination of continued treatment for CAD for the subject, the system comprising:
one or more computer readable storage devices configured to store a plurality of computer executable instructions; and
one or more hardware computer processors in communication with the one or more computer readable storage devices and configured to execute the plurality of computer executable instructions in order to cause the system to:
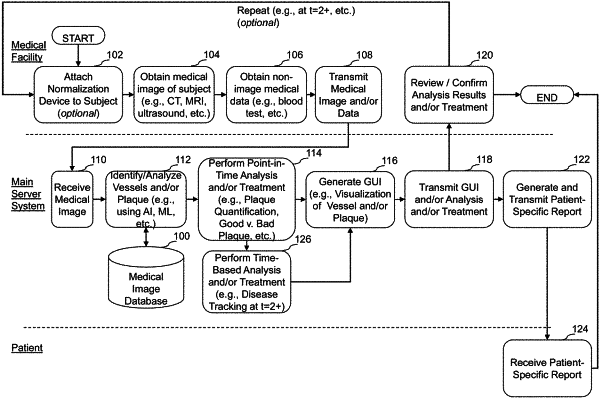
access a first set of quantified plaque parameters for one or more regions of one or more coronary arteries of a subject, wherein the first set of quantified plaque parameters are generated from analyzing a first medical image of the subject comprising the one or more regions of the one or more coronary arteries, the first medical image of the subject obtained at a first point in time, wherein the first set of quantified plaque parameters comprises total plaque volume, non-calcified plaque volume, and calcified plaque volume identified in the one or more regions of the one or more coronary arteries of the subject at the first point in time;
access a second medical image of the subject, the second medical image of the subject obtained at a second point in time after treatment or deferring treatment the subject with a treatment for CAD, the treatment comprising one or more of pharmaceutical treatment, lifestyle change, or surgery, the second point in time being later than the first point in time, the second medical image of the subject comprising the one or more regions of the one or more coronary arteries of the subject;
identify the one or more regions of the one or more coronary arteries of the subject from the first medical image in the second medical image;
analyze the second medical image to generate a second set of quantified plaque parameters for the one or more regions of the one or more coronary arteries of the subject, wherein the second set of quantified plaque parameters comprises total plaque volume, non-calcified plaque volume, and calcified plaque volume identified in the one or more regions of the one or more coronary arteries of the subject at the second point in time;
determine a post-treatment change in total plaque volume between the first medical image and the second medical image, wherein an increase in total plaque volume is indicative of progression of CAD;
determine a post-treatment change in non-calcified plaque volume between the first medical image and the second medical image, wherein an increase in non-calcified plaque volume is indicative of progression of CAD;
determine a post-treatment change in calcified plaque volume between the first medical image and the second medical image, wherein an increase in calcified plaque volume is indicative of stabilization of CAD; and
generate a graphical representation comprising the determined post-treatment change in total plaque volume, non-calcified plaque volume, and calcified plaque volume for the one or more regions of the one or more coronary arteries, wherein the graphical representation is configured to facilitate determination of a state of CAD progression, regression, or stabilization based at least in part on the determined change in post-treatment total plaque volume, non-calcified plaque volume, and calcified plaque volume, and wherein the determination of the state of CAD progression, regression, or stabilization is configured to facilitate determination of continued treatment for CAD for the subject.
|