|
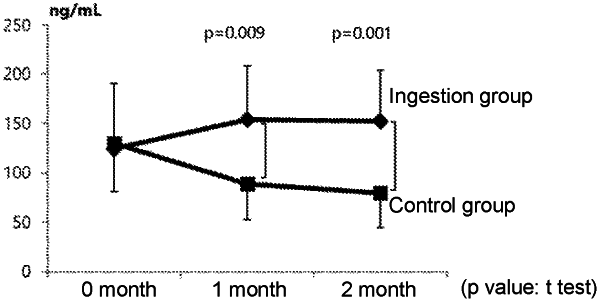
1. A method of enhancing a breast milk component in a pregnant or lactating woman in need thereof, the method comprising a step of administering a composition comprising a therapeutically effective amount of Bifidobacterium breve to the woman; wherein the composition optionally further comprises a therapeutically effective amount of a bacterium of the species Bifidobacterium longum, wherein the breast milk component is selected from the group consisting of a heat-shock protein, a chemokine, an interferon, a lectin, and combinations thereof.
|