| CPC C07D 471/04 (2013.01) [A61K 31/453 (2013.01); A61K 31/4545 (2013.01); A61K 31/4725 (2013.01); A61K 31/496 (2013.01); A61K 31/536 (2013.01); A61K 31/538 (2013.01); A61K 31/551 (2013.01); A61K 31/553 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); A61P 37/00 (2018.01); C07D 405/04 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01)] | 8 Claims |
|
1. A pharmaceutical composition comprising a pharmaceutically acceptable carrier, diluent, or excipient and a compound having the formula (Ia1′):
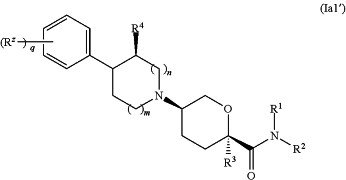 or a pharmaceutically acceptable salt thereof,
wherein:
R1 is aryl-C1-4 alkylene, heteroaryl-C1-4 alkylene, aryl, or heteroaryl;
wherein the heteroaryl or heteroaryl portion of heteroaryl-C1-4 alkylene has 1, 2, or 3 ring heteroatoms independently selected from the group consisting of nitrogen, oxygen, and sulfur; and
wherein the aryl portion of aryl-C1-4 alkylene, heteroaryl portion of heteroaryl-C1-4 alkylene, aryl, or heteroaryl is optionally substituted with 1, 2, 3, 4, or 5 independently selected RX substituents;
R2 is H, C1-8 alkyl, C3-8 cycloalkyl-C1-4 alkylene, aryl-C1-4 alkylene, heteroaryl-C1-4 alkylene, C3-8 cycloalkyl, aryl, or heteroaryl;
wherein the heteroaryl or heteroaryl portion of heteroaryl-C1-4 alkylene has 1, 2, or 3 ring heteroatoms independently selected from the group consisting of nitrogen, oxygen, and sulfur; and
wherein the aryl portion of aryl-C1-4 alkylene, heteroaryl portion of heteroaryl-C1-4 alkylene, aryl, or heteroaryl is optionally substituted with 1, 2, 3, or 4 independently selected RX substituents; or
R1 and R2 together with the nitrogen atom to which they are attached, form a monocyclic 6- to 11-membered heterocyclyl, a fused bicyclic 6- to 11-membered heterocyclyl, a monocyclic 6- to 11-membered heteroaryl, or a fused bicyclic 6- to 11-membered heteroaryl;
wherein the monocyclic 6- to 11-membered heterocyclyl, fused bicyclic 6- to 11-membered heterocyclyl, monocyclic 6- to 11-membered heteroaryl, or fused bicyclic 6- to 11-membered heteroaryl is optionally substituted with 1, 2, 3, or 4 independently selected RX substituents;
each Rx is independently halogen, —CN, —Rc, —X1C(O)NRaRb, —X1C(O)ORa, —X1NRaRb, —X1ORa, —C(O)Ra, —C(O)NRaRb, —C(O)ORa, —NRaRb, —NRbC(O)Ra, —NRaC(O)NRaRb, —NRbC(O)ORe, —ORa, —OX1C(O)NRaRb, —OX1C(O)ORa, —OX1NRaRb, —OX1ORa, —OC(O)NRaRb, —SF5, —S(O)2NRaRb, phenyl, or 5- or 6-membered heteroaryl, wherein each phenyl and 5- or 6-membered heteroaryl is optionally and independently substituted with 1, 2, or 3 substituents independently selected from the group consisting of halogen, C1-4 alkyl, C1-4 haloalkyl, OH, C1-4 alkoxy, and C1-4 haloalkoxy; or
two vicinal Rx, together with the carbon atoms to which they are attached, form a fused 5- or 6-membered carbocyclyl;
each Ra is independently H, C1-8 alkyl, or C1-8 haloalkyl;
each Rb is independently H, C1-8 alkyl, or C1-8 haloalkyl; or
each Ra and Rb, together with the nitrogen atom to which they are attached, independently forms a 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl;
wherein each 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl independently has 0, 1, or 2 additional ring heteroatoms independently selected from the group consisting of nitrogen, oxygen, and sulfur; and
wherein each 5- or 6-membered heterocyclyl is optionally and independently substituted with 1 oxo substituent;
each Rc is independently C1-8 alkyl, C1-8 haloalkyl, or C3-6 cycloalkyl;
R3 is H, C1-8 alkyl, C3-8 cycloalkyl-C1-4 alkylene, or C3-8 cycloalkyl, wherein the C1-8 alkyl, C3-8 cycloalkyl portion of C3-8 cycloalkyl-C1-4 alkylene, or C3-8 cycloalkyl is optionally substituted with 1, 2, or 3 independently selected Ry substituents;
R4 is H, C1-8 alkyl, or C(O)OH, wherein the C1-8 alkyl is optionally substituted with 1 or 2 independently selected Ry substituents;
each Ry is independently halogen, —CN, —Rf, —C(O)Rd, —C(O)NRdRe, —C(O)ORd, —NRdRe, —NReC(O)Rd, —NRdC(O)NRdRe, —NReC(O)ORf, —ORd, —OC(O)NRdRe, or —S(O)2NRdRe;
each Rd is independently H, C1-8 alkyl, or C1-8 haloalkyl;
each Re is independently H, C1-8 alkyl, or C1-8 haloalkyl; or
each Rd and Re, together with the nitrogen atom to which they are attached, independently forms a 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl;
wherein each 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl independently has 0, 1, or 2 additional ring heteroatoms independently selected from the group consisting of nitrogen, oxygen, and sulfur;
each Rf is independently C1-8 alkyl, C1-8 haloalkyl, or C3-6 cycloalkyl;
each Rz is independently halogen, —CN, —Ri, —X1C(O)NRgRh, —X1NRgRh, —X1NRhC(O)Rg, —C(O)Rg, —C(O)NRgRh, —C(O)ORg, —NRgRh, —NHCH2Rj, —NRhC(O)Rg, —NRgC(O)NRgRh, —NRhC(O)ORi, —NH(pyrrolinyl), —NH(tetrahydrofuranyl), —NH(piperidinyl), —NH(tetrahydropyranyl), —NH(morpholinyl), —ORg, —OC(O)NRgRh, —S(O)2NRgRh, or tetrazolyl;
each Rg is independently H, C1-8 alkyl, Cis haloalkyl, or C3-6 cycloalkyl;
each Rh is independently H, C1-8 alkyl, Cis haloalkyl, or C3-6 cycloalkyl; or
each Rg and Rh, together with the nitrogen atom to which they are attached, independently forms a 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl;
wherein each 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl independently has 0, 1, or 2 additional ring heteroatoms independently selected from the group consisting of nitrogen, oxygen, and sulfur; and
wherein each 5- or 6-membered heterocyclyl is optionally and independently substituted with 1 or 2 oxo substituents;
each Ri is independently C1-8 alkyl, C1-8 haloalkyl, or C3-6 cycloalkyl;
each Rj is independently C3-6 cycloalkyl, pyrrolinyl, tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, or morpholinyl;
each X1 is independently C1-4 alkylene;
m is 1;
n is 1; and
q is 1, 2, 3, 4, or 5.
|