| CPC A61N 1/36121 (2013.01) [A61N 1/36062 (2017.08); A61N 1/36135 (2013.01); A61N 1/36175 (2013.01); A61N 1/36192 (2013.01); A61N 1/36196 (2013.01)] | 20 Claims |
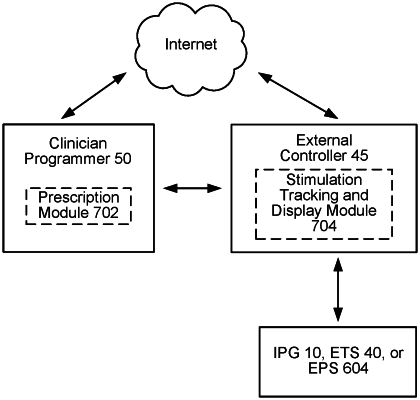
|
1. A method for providing stimulation to a patient using an implantable stimulator device and an external device in communication with the implantable stimulator device, comprising:
determining stimulation parameters for the patient to address a symptom of the patient;
providing a first option on a graphical user interface of the external device to schedule scheduled boluses of stimulation for the patient, wherein each scheduled bolus comprises a first duration during which stimulation is applied to the patient in accordance with the stimulation parameters, wherein the scheduled boluses are separated by off times of a second duration when no stimulation is provided to the patient;
providing a second option on the graphical user interface of the external device to receive from the patient at a first time an input to immediately provide an additional unscheduled bolus of stimulation in accordance with the stimulation parameters; and
providing, using the implantable stimulator device, at least the scheduled boluses to neural tissue of the patient according to the schedule.
|