| CPC C07K 16/2875 (2013.01) [A61K 47/42 (2013.01); A61K 47/6817 (2017.08); A61K 47/6845 (2017.08); A61K 47/6889 (2017.08); C07K 5/06052 (2013.01); C07K 2317/76 (2013.01)] | 19 Claims |
|
1. A compound comprising Formula (VIII) or (IX), wherein the compound is an anti-CD70 antibody conjugated to a dolastatin, wherein the conjugation occurs via a non-natural amino acid in the anti-CD70 antibody, and Formula (VIII) or (IX) has the following structure:
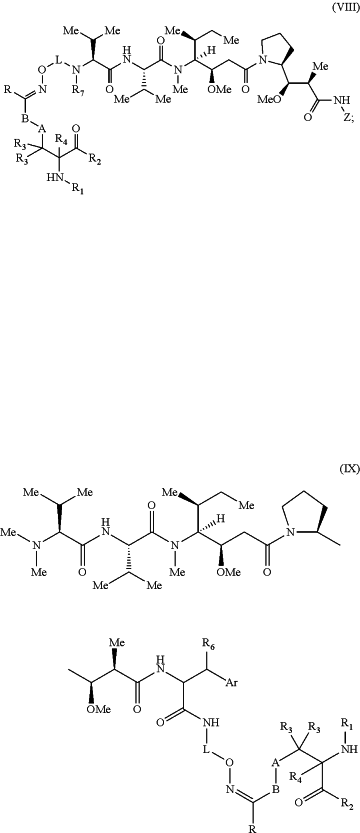 wherein:
A is optional, and when present is lower alkylene, substituted lower alkylene, lower cycloalkylene, substituted lower cycloalkylene, lower alkenylene, substituted lower alkenylene, alkynylene, lower heteroalkylene, substituted heteroalkylene, lower heterocycloalkylene, substituted lower heterocycloalkylene, arylene, substituted arylene, heteroarylene, substituted heteroarylene, alkarylene, substituted alkarylene, aralkylene, or substituted aralkylene;
B is optional, and when present is a linker selected from the group consisting of lower alkylene, substituted lower alkylene, lower alkenylene, substituted lower alkenylene, lower heteroalkylene, substituted lower heteroalkylene, —O—, -O-(alkylene or substituted alkylene)-, -S-, -S-(alkylene or substituted alkylene)-, —S(O)k— wherein k is 1, 2, or 3, —S(O)k(alkylene or substituted alkylene)-, —C(O)—, —C(O)-(alkylene or substituted alkylene)-, —C(S)—, —C(S)-(alkylene or substituted alkylene)-, —N(R′)—, —NR′-(alkylene or substituted alkylene)-, —C(O)N(R′)-, —CON(R′)-(alkylene or substituted alkylene)-, —CSN(R′)—, —CSN(R′)-(alkylene or substituted alkylene)-, —N(R′)CO-(alkylene or substituted alkylene)-, —N(R′)C(O)O—, —S(O)kN(R′)-, —N(R′)C(O)N(R′)-, —N(R′)C(S)N(R′)-, —N(R′)S(O)kN(R′)-, —N(R′)—N═, —C(R′)═N-, —C(R′)═N—N(R′)-, —C(R′)=N-N═, —C(R′)2-N═N-, and —C(R′)2-N(R′)-N(R′)-, wherein each R′ is independently H, alkyl, or substituted alkyl;
R is H, alkyl, substituted alkyl, cycloalkyl, or substituted cycloalkyl;
R1 is a polypeptide, wherein the polypeptide comprises a heavy chain variable region having the amino acid sequence of SEQ ID NO: 1;
R2 is a polypeptide;
R3 and R4 are each independently H, halogen, lower alkyl, or substituted lower alkyl, or R3 and R4 or two R3 groups optionally form a cycloalkyl or a heterocycloalkyl;
Z has the structure of:
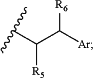 wherein:
R5 is H, COR8, C1-C6 alkyl or thiazole;
R8 is OH;
R6 is OH or H; and
Ar is phenyl or pyridine;
R7 is H or C1-C6 alkyl; and
L is a linker selected from the group consisting of -alkylene-, -alkylene-C(O)—, -(alkylene-O)n-alkylene-, -(alkylene-O)n-alkylene-C(O)—, -(alkylene-O)n—(CH2)n′—NHC(O)—(CH2)n″—C(Me)2-S—S—(CH2)n′″—NHC(O)-(alkylene-O)n″″-alkylene-, -(alkylene-O)n-alkylene-W—, -alkylene-C(O)—W—, -(alkylene-O)n-alkylene-U-alkylene-C(O)—, and -(alkylene-O)n-alkylene-U-alkylene-;
wherein:
W has the structure of:
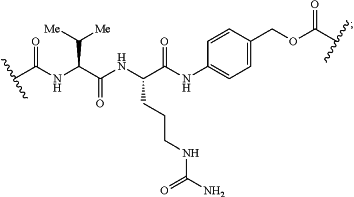 U has the structure of:
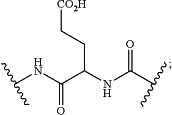 and
each n, n′, n″, n′″ and n″″ is independently an integer greater than or equal to one;
or an active metabolite, or a pharmaceutically acceptable prodrug or solvate thereof.
|