| CPC A61B 18/1206 (2013.01) [A61B 18/10 (2013.01); A61B 18/1492 (2013.01); A61F 7/12 (2013.01); A61B 2017/00075 (2013.01); A61B 2018/00404 (2013.01); A61B 2018/00434 (2013.01); A61B 2018/00505 (2013.01); A61B 2018/00511 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00642 (2013.01); A61B 2018/00648 (2013.01); A61B 2018/00672 (2013.01); A61B 2018/00678 (2013.01); A61B 2018/00702 (2013.01); A61B 2018/00761 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00797 (2013.01); A61B 2018/00815 (2013.01); A61B 2018/00821 (2013.01); A61B 2018/00863 (2013.01); A61B 2018/00875 (2013.01); A61F 2007/0093 (2013.01); A61F 2007/126 (2013.01)] | 20 Claims |
|
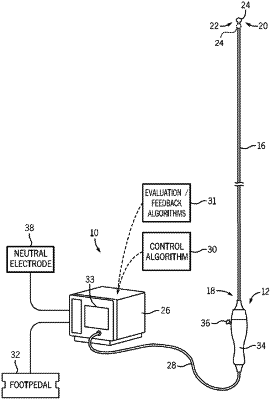
1. A method for providing feedback related to a neuromodulation treatment, the method comprising:
determining an unexpected or aberrant condition associated with the neuromodulation treatment occurred during administration of the neuromodulation treatment;
based on determining the unexpected or aberrant condition occurred, obtaining a set of treatment data corresponding to the neuromodulation treatment;
evaluating the set of treatment data in view of one or more criteria to determine appropriate feedback; and
providing the appropriate feedback based on the evaluation.
|