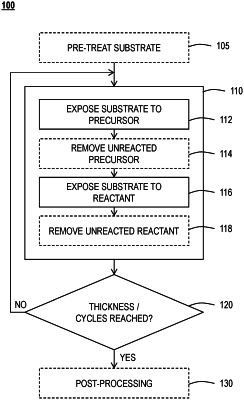
| CPC C23C 16/45553 (2013.01) [C07F 7/003 (2013.01); C07F 7/025 (2013.01); C07F 7/2224 (2013.01); C07F 7/2284 (2013.01); C07F 7/30 (2013.01); C23C 16/18 (2013.01); C23C 16/45534 (2013.01)] | 16 Claims |
|
1. A method of forming a metal film, the method comprising exposing a metal halide film to a reducing agent, the reducing agent comprising one or more of
a. a group IV element containing heterocyclic compound having the formula,
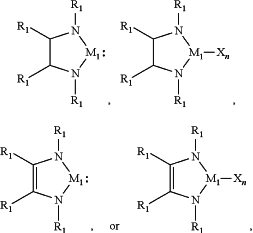 wherein, each of R1 is independently a C1-C6 alkyl, alkenyl or alkynyl group or C1-C8 cycloalkyl or aryl group, X is a halogen, n is a number in a range of from 1 to 4, M1 is selected from a group consisting of Ge and Sn;
b. a radical initiator having a formula ((R2)3Si)3Si-H, where each R2 is independently H, C1-C6 alkyl, alkenyl or alkynyl group or C1-C8 cycloalkyl or aryl group, a halogen;
c. a diborene species having the formula
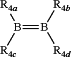 wherein, each of R4a, R4b, R4c and R4d are independently a C1-C6 alkyl group, or a strong electron-donating group, the strong electron-donating group comprises one or more of an N-heterocyclic carbene, an alkyl silane, an alkyl amide or an organic alcohol; or
d. a Sn(II) compound to reduce the metal film and form Sn(IV) compounds, wherein the Sn(II) compounds has a structure according to formula (X), (XI), (XII), (XIII) or (XIV),
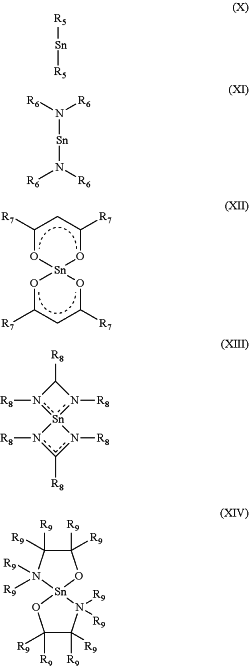 wherein, each of R5 are independently selected from a group consisting of Cp, Cp* and Cp-Rz, where Rz is a C1-C6 alkyl group and z is 1 to 5,
wherein, each of R6 are independently selected from a group consisting of Si(Me)3, Me, Et, iPr and tBu,
wherein, each of R7 are independently selected from a group consisting of Me, CF3 and tBu,
wherein, each of R8 are independently selected from a group consisting of Me, iPr and tBu, and
wherein, each of R9 are independently selected from a group consisting of H, Me, iPr and tBu.
|