| CPC C12N 5/0018 (2013.01) [C12N 5/0682 (2013.01); C12N 15/79 (2013.01); C12N 2500/32 (2013.01); C12N 2500/60 (2013.01)] | 13 Claims |
|
1. A method for selecting a cell culture medium with reduced impurities for use in cell culture to improve cell culture performance, wherein the cell culture comprises at least one recombinant eukaryotic cell that can express a recombinant protein, the method comprising:
(a) providing a cell culture medium comprising a 4-hydroxyethyl piperazine ethanesulfonic acid (HEPES) buffer;
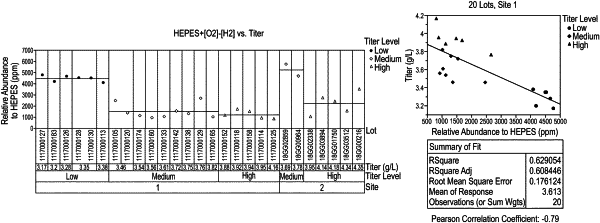
(b) analyzing the cell culture medium comprising the HEPES buffer to determine the amount of HEPES related impurities HEPES+[O2]−[H2] and HEPES-[CH4] present in the cell culture medium;
(c) selecting the cell culture medium comprising the HEPES buffer for use in cell culture if the cell culture medium comprising the HEPES buffer is determined to have less than 4000 μmol of HEPES+[O2]−[H2] per mole of total HEPES, and less than 400 μmol of HEPES-[CH4] per mole of total HEPES;
wherein the use of the cell culture medium comprising the HEPES buffer having less than 4000 μmol of HEPES+[O2]−[H2] per mole of total HEPES, and less than 400 μmol of HEPES-[CH4] per mole of total HEPES improves cell culture performance, as compared to cell culture performance in non-HEPES related impurity reduced media.
|
|
2. The method of claim 1, wherein the improved cell culture performance comprises at least one selected from the group consisting of improved protein titer and cell growth.
|
|
3. The method of claim 1, wherein the eukaryotic cell is selected from the group consisting of mammalian cell, avian cell, insect cell, and yeast cell.
|
|
4. The method of claim 1, wherein the eukaryotic cell is selected from the group consisting of CHO, COS, retinal cell, Vero, CV1, kidney, HeLa, HepG2, WI38, MRC 5, Colo25, HB 8065, HL-60, lymphocyte, A431, CV-1, U937, 3T3, L cell, C127 cell, SP2/0, NS-0, MMT cell, stem cell, tumor cell, and a cell line derived from an aforementioned cell.
|
|
5. The method of claim 1, wherein the eukaryotic cell is a CHO cell.
|