| CPC C12N 15/86 (2013.01) [A61P 35/00 (2018.01); C07K 16/2818 (2013.01); C07K 16/2827 (2013.01); C12N 7/00 (2013.01); C12N 2710/10332 (2013.01); C12N 2710/10343 (2013.01)] | 20 Claims |
|
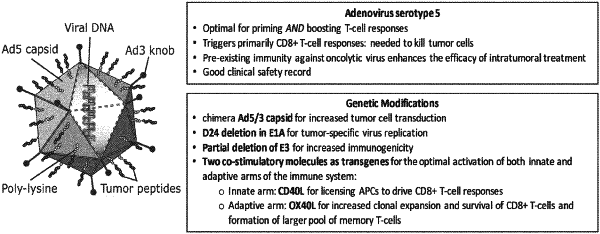
1. A modified adenovirus comprising:
at least one polypeptide attached covalently or non- covalently onto the viral capsid without having been genetically encoded by said adenovirus, wherein the at least one polypeptide comprises:
i) VFGIELMEVDPIGHLYIFAT [SEQ ID NO:1];
ii) YLAMPFATPMEAELARRSLA [SEQ ID NO:2]; or
iii) a polypeptide that is at least 90% identical to SEQ ID NO: 1 or SEQ ID NO: 2;
a deletion of the 14.7 k gene;
a transgene encoding CD40L; and
a transgene encoding OX40L situated in the E3 region of the modified adenovirus.
|