|
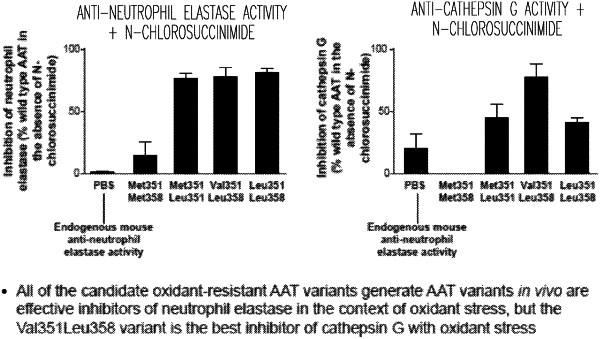
8. A method to inhibit cathepsin G and neutrophil elastase under conditions of oxidative damage or oxidative stress in a lung of a mammal having emphysema, COPD, respiratory distress syndrome or fibrotic interstitial lung disease comprising: administering to a pleura of the mammal, an amount of an adeno-associated viral gene therapy vector comprising an expression cassette coding for a human oxidation-resistant alpha 1-antitrypsin (AAT) that has a leucine at position 358 and a valine at position 351, wherein expression of the oxidation-resistant AAT from the vector in the lung of the mammal results in inhibition of cathepsin G and neutrophil elastase under conditions of the oxidative damage or stress in the lung as a result of increased levels of the oxidation-resistant AAT in the mammal after administration, and wherein the mammal prior to administration of the vector has serum AAT levels of less than 11 μM.
|