| CPC C08G 61/02 (2013.01) [C08G 61/12 (2013.01); C08G 2261/314 (2013.01); C08G 2261/3222 (2013.01); C08G 2261/418 (2013.01); C08G 2261/70 (2013.01)] | 36 Claims |
|
1. A composition comprising:
a) a copolymer prepared by a method comprising polymerizing in the presence of a metathesis catalyst:
i) a first monomer, wherein each instance of the first monomer is independently of the formula:
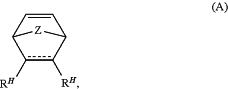 or salt thereof, wherein:
each instance of Z is independently a single bond, C(RP)2, or O;
each instance of RP is independently hydrogen, halogen, or substituted or unsubstituted, C1-6 alkyl;
each instance of
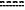 is independently a single or double bond; is independently a single or double bond;each instance of RH is independently hydrogen, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, —ORa, —OCN, —OC(═O)Ra, —OC(═S)Ra, —OC(═O)ORa, —OC(═O)N(Ra)2, —OS(═O)Ra, —OS(═O)ORa, —OS(═O)N(Ra)2, —OS(═O)2Ra, —OS(═O)2ORa, —OS(═O)2N(Ra)2, —OSi(Ra)3, —OSi(Ra)2(ORa), —OSi(Ra)(ORa)2, —OSi(ORa)3, oxo, —N(Ra)2, —N═C(Ra)2, ═NRa, —NC, —NCO, —N3, —NO2, —NRaC(═O)Ra, —NRaC(═O)ORa, —NRaC(═O)N(Ra)2, —NRaS(═O)Ra, —NRaS(═O)ORa, —NRaS(═O)N(Ra)2, —NRaS(═O)2Ra, —NRaS(═O)2ORa, —NRaS(═O)2N(Ra)2, —SRa, —SCN, —S(═O)Ra, —S(═O)ORa, —S(═O)N(Ra)2, —S(═O)2Ra, —S(═O)2ORa, —S(═O)2N(Ra)2, —SeRa, halogen, —CN, —C(═NRa)Ra, —C(═NRa)ORa, —C(═NRa)N(Ra)2, —C(═O)Ra, —C(═O)ORa, —C(═O)SRa, —C(═S)ORa, or —C(═O)N(Ra)2;
or the two instances of RH of one or more instances of
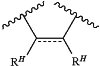 are joined with the intervening carbon atoms to independently form a substituted or unsubstituted, monocyclic carbocyclic ring, substituted or unsubstituted, monocyclic heterocyclic ring, substituted or unsubstituted, monocyclic aryl ring, or substituted or unsubstituted, monocyclic heteroaryl ring; and
each instance of Ra is independently hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted, monocyclic carbocyclyl, substituted or unsubstituted, monocyclic heterocyclyl, substituted or unsubstituted, monocyclic aryl, substituted or unsubstituted, monocyclic heteroaryl, a nitrogen protecting group when attached to a nitrogen atom, an oxygen protecting group when attached to an oxygen atom, or a sulfur protecting group when attached to a sulfur atom, or two instances of Ra are joined to form substituted or unsubstituted heterocyclyl or substituted or unsubstituted heteroaryl;
ii) a second monomer, wherein each instance of the second monomer is independently of the formula:
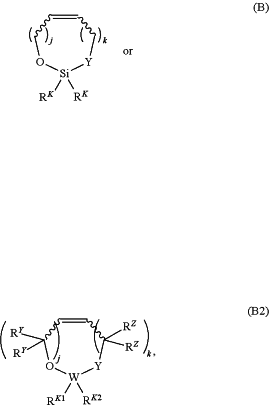 or a salt thereof, wherein:
each instance of Y is independently O or C(RQ)2;
each instance of RQ is independently hydrogen, halogen, or substituted or unsubstituted, C1-6 alkyl;
each instance of RK is independently hydrogen, halogen, substituted or unsubstituted, C1-10 alkyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or —ORN;
each instance of RN is independently hydrogen, substituted or unsubstituted acyl, substituted or unsubstituted, C1-10 alkyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an oxygen protecting group;
each instance of j is independently 1, 2, or 3;
in Formula (B), each instance of k is independently 0, 1, 2, or 3; and
in Formula (B2):
W is silicon;
each instance of RY is independently hydrogen, halogen, or substituted or unsubstituted, C1-6 alkyl;
each instance of RZ is independently hydrogen, halogen, or substituted or unsubstituted, C1-6 alkyl;
RK1 is hydrogen, halogen, substituted or unsubstituted, C1-10 alkyl, substituted or unsubstituted, C2-10 alkenyl, substituted or unsubstituted, C2-10 alkynyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, -LK1-(substituted or unsubstituted carbocyclyl), -LK1-(substituted or unsubstituted heterocyclyl), -LK1-(substituted or unsubstituted aryl), -LK1-(substituted or unsubstituted heteroaryl), or —ORN1;
LK1 is —O—, substituted or unsubstituted, C1-10 alkylene, substituted or unsubstituted, C2-10 heteroalkylene, substituted or unsubstituted carbocyclylene, substituted or unsubstituted heterocyclylene, substituted or unsubstituted arylene, substituted or unsubstituted heteroarylene, or a combination thereof;
RN1 is independently hydrogen, substituted or unsubstituted acyl, substituted or unsubstituted, C1-10 alkyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an oxygen protecting group;
RK2 is halogen, substituted or unsubstituted, C2-10 alkenyl, substituted or unsubstituted, C2-10 alkynyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted heteroaryl, -LK2-(substituted or unsubstituted carbocyclyl), -LK2-(substituted or unsubstituted heterocyclyl), -LK2-(substituted or unsubstituted aryl), or —ORN2;
LK2 is —O—, substituted or unsubstituted, C1-10 alkylene, substituted or unsubstituted, C2-10 heteroalkylene, substituted or unsubstituted carbocyclylene, substituted or unsubstituted heterocyclylene, substituted or unsubstituted arylene, substituted or unsubstituted heteroarylene, or a combination thereof;
RN2 is independently hydrogen, substituted or unsubstituted acyl, substituted or unsubstituted, C1-10 alkyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an oxygen protecting group;
or RK1 and RK2 are joined with the intervening atom to form substituted or unsubstituted carbocyclyl or substituted or unsubstituted heterocyclyl; and
each instance of k is independently 1, 2, or 3;
iii) optionally a third monomer, wherein the third monomer is different from the first monomer and the second monomer; and
iv) optionally a reprocessing catalyst; and
b) optionally a reprocessing catalyst;
wherein the reprocessing catalyst is an unsubstituted C2-20 alkanoic acid, a sulfonic acid, a sulfinic acid, a phosphoric acid, a phosphonic acid, a Lewis acid, a Lewis base, a urea, a thiourea, a carbamate, or a thiocarbamate;
provided that:
the reprocessing catalyst is not an ammonium halide; and
the composition comprises at least one of the reprocessing catalyst of iv) and the reprocessing catalyst of b).
|