| CPC C07K 16/2878 (2013.01) [A61K 47/6849 (2017.08); A61P 17/00 (2018.01); A61P 35/04 (2018.01); A61P 37/02 (2018.01); A61P 37/06 (2018.01); C12N 15/63 (2013.01); A61K 2039/505 (2013.01); C07K 2317/24 (2013.01); C07K 2317/565 (2013.01); C07K 2317/71 (2013.01); C07K 2317/76 (2013.01); C07K 2317/92 (2013.01)] | 13 Claims |
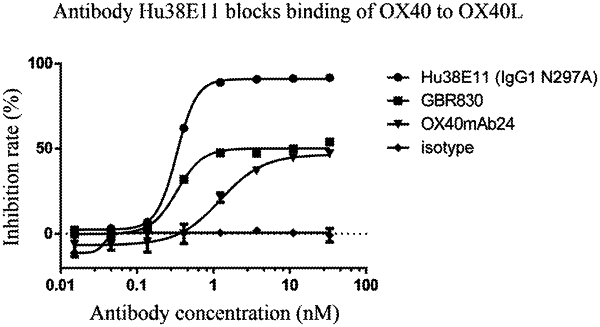
|
1. An isolated anti-OX40 antibody, comprising
(1) heavy chain complementary determining regions (HCDRs), HCDR1, HCDR2 and HCDR3, wherein the HCDR1 comprises the amino acid sequence as set forth in SEQ ID NO: 11, the HCDR2 comprises the amino acid sequence as set forth in SEQ ID NO: 12, and the HCDR3 comprises the amino acid sequence as set forth in SEQ ID NO: 13;
(2) light chain complementary determining regions (LCDRs), LCDR1, LCDR2 and LCDR3, wherein the LCDR1 comprises the amino acid sequence as set forth in SEQ ID NO: 14, the LCDR2 comprises the amino acid sequence as set forth in SEQ ID NO: 15, and the LCDR3 comprises the amino acid sequence as set forth in SEQ ID NO: 16; and
(3) Fc region variant, which is human IgG1 N297A;
wherein the amino acid residues in the Fc region are numbered in accordance with the EU numbering system.
|