| CPC C07D 498/08 (2013.01) [A61P 35/00 (2018.01); C07D 251/66 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 405/12 (2013.01); C07D 409/12 (2013.01); C07D 413/14 (2013.01); C07D 487/04 (2013.01); C07D 495/04 (2013.01)] | 18 Claims |
|
1. A compound selected from:
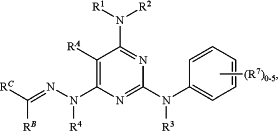 or a pharmaceutically acceptable salt thereof, wherein:
R1 is selected from H and C1-6 alkyl optionally substituted with ORa2;
R2 is C1-6 alkyl optionally substituted with ORa2; or
R1 and R2 together form a 4-7 membered heterocycloalkyl, which is optionally substituted with 1, 2, or 3 substituents independently selected R8;
each R8 is selected from C1-6 alkyl and ORa2;
Ra2 is selected from H and C1-6 alkyl;
R3 is selected from H, C1-6 alkyl, and C1-6 haloalkyl;
R4 is selected from H, C1-6 alkyl, and C1-6 haloalkyl;
each R7 is selected from halo, CN, NO2, C1-6 alkyl, C1-6 haloalkyl, ORa1, C(O)Rb1, C(O)NRc1Rd1, C(O)ORa1, NRc1Rd1, NRc1C(O)Rb1, NRc1C(O)ORa1, NRc1S(O)2Rb1, S(O)2Rb1, and S(O)2NRc1Rd1;
Ra1, Rc1, and Rd1 are each independently selected from H, C1-6 alkyl, and C1-4 haloalkyl;
each Rb1 is independently selected from C1-6 alkyl and C1-4 haloalkyl;
RA is selected from H, C1-6 alkyl, and C1-6 haloalkyl;
RB is selected from H, C1-6 alkyl, and C1-6 haloalkyl;
RC is selected from C6-10 aryl, 5-membered heteroaryl, and 7-10 membered heteroaryl, each of which is optionally substituted with 1, 2, or 3 substituents independently selected from halo, CN, NO2, C1-6 alkyl, C1-6 haloalkyl, ORa3, NRc3Rd3 NRc3C(O)Rb3, and NRc3S(O)2Rb3;
Ra3, Rc3, and Rd3 are each independently selected from H, C1-6 alkyl, and C1-4 haloalkyl; and
each Rb3 is independently selected from C1-6 alkyl and C1-4 haloalkyl.
|