| CPC C07D 471/04 (2013.01) [A61K 9/0014 (2013.01); C07D 213/64 (2013.01); C07D 213/643 (2013.01); C07D 213/71 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 409/12 (2013.01); C07D 409/14 (2013.01); C07D 413/12 (2013.01); C07D 417/12 (2013.01); C07F 5/027 (2013.01)] | 23 Claims |
|
1. A compound of formula (I):
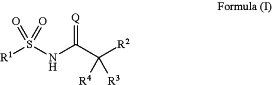 or a pharmaceutically acceptable salt or solvate thereof, wherein:
Q is selected from O or S;
R1 is a saturated or unsaturated hydrocarbyl group, wherein the hydrocarbyl group may be straight-chained or branched, or be or include cyclic groups, wherein the hydrocarbyl group may optionally be substituted, and wherein the hydrocarbyl group may optionally include one or more heteroatoms N, O or S in its carbon skeleton;
R2 is a 6-membered cyclic group substituted at the α and α′ positions, wherein the substituent at the α-position is a monovalent heterocyclic group or a monovalent aromatic group, wherein a ring atom of the heterocyclic or aromatic group is directly attached to the α-ring atom of the cyclic group, wherein the heterocyclic or aromatic group may optionally be substituted, wherein the substituent at the α′-position comprises at least one carbon atom, and wherein the cyclic group may optionally be further substituted;
R3 is hydrogen, halogen, —OH, —NH2, —CN, —R5, —OR5, —NHR5 or —N(R5)2;
R4 is hydrogen, halogen, —OH, —NH2, —CN, —R5, —OR5, —NHR5 or —N(R5)2; or
R3 and R4 together with the carbon atom to which they are attached may form a 3- to 7-membered saturated or unsaturated cyclic group, wherein the cyclic group may optionally be substituted; and
R5 is independently optionally substituted C1-C4 alkyl.
|