| CPC C07D 405/14 (2013.01) [C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 409/14 (2013.01); C07D 413/04 (2013.01); C07D 413/14 (2013.01); C07D 417/04 (2013.01); C07D 417/14 (2013.01)] | 15 Claims |
|
1. A compound having a structural Formula (I):
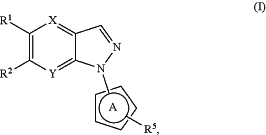 or a pharmaceutically acceptable salt thereof, wherein:
X is selected from N, C—H, C—F, and C—Cl;
Y is selected from N, C—H, C—F, and C—Cl;
R1 is selected from H, F, Cl, CN, —(C1-C3)alkyl, —O(C1-C3)alkyl, —(C1-C3)haloalkyl, —O(C1-C3)haloalkyl, and —(C3-C6)cycloalkyl;
R2 is a moiety selected from:
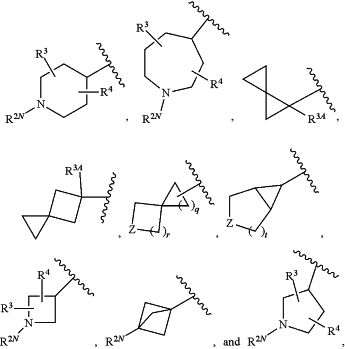 wherein:
q is 1, 2, or 3;
r is 1 or 2;
t is 1 or 2;
Z is selected from O and N(R2N);
R2N is selected from H, (C1-C6)alkyl, —(C1-C6)haloalkyl, (C1-C6)alkyl-OH, (C1-C6)alkyl-CN, —S(O)2(C1-C6)alkyl, —(C1-C6)alkyl-S(O)2(C1-C6)alkyl, C(O)O(C1-C6)alkyl, C(O)NH2, C(O)NH(C1-C6)alkyl, —C(O)N((C1-C6)alkyl)2, (C1-C6)alkyl-O—(C1-C6)alkyl, oxetanyl which is optionally substituted with R2A, furanyl which is optionally substituted with 1 or 2 groups selected from OH and R2A, pyranyl which is optionally substituted with 1 or 2 groups selected from OH and R2A, and
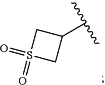 each R2A is independently selected from H and —(C1-C4)alkyl;
R3 is selected from H, F, Cl, (C1-C4)alkyl, (C1-C6)haloalkyl, and (C1-C6)alkyl-OH;
R3A is selected from H and CN;
R4 is selected from H, F, Cl, (C1-C4)alkyl, (C1-C6)haloalkyl, and (C1-C6)alkyl-OH;
ring A is 5-membered heteroaryl group comprising 1, 2, or 3 ring heteroatoms selected from N, O, and S;
R5 is selected from H, (C1-C6)alkyl, (C1-C6)haloalkyl, (C3-C6)cycloalkyl, (C3-C6)cycloalkyl substituted with (C1-C4)alkyl, S(O)2(C3-C6)cycloalkyl, C(O)N(R5A)2, C(O)OR5A, phenyl, heteroaryl, heterocycloalkyl and
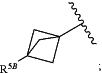 and
each R5A is independently selected from H and —(C1-C4)alkyl; and
R5B is selected from H, (C1-C4)alkyl, (C1-C4)haloalkyl, (C1-C4)alkyl-O—(C1-C4)alkyl, CN, S(O)2(C3-C6)cycloalkyl, C(O)N(R5A)2, and C(O)OR5A.
|