| CPC C07D 403/08 (2013.01) [A61P 31/20 (2018.01); C07D 231/38 (2013.01); C07D 401/08 (2013.01); C07D 403/04 (2013.01); C07D 403/10 (2013.01); C07D 403/12 (2013.01); C07D 405/04 (2013.01); C07D 413/04 (2013.01); C07D 413/08 (2013.01); C07D 417/08 (2013.01)] | 5 Claims |
|
1. A method for synthesizing a compound (VI-5)
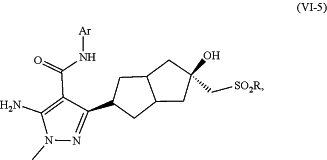 or a pharmaceutically acceptable salt thereof,
wherein
Ar is optionally substituted phenyl; and
R is alkyl;
the method comprising:
a) condensing a carboxylic acid ester or chloride (I-1) with a compound of formula (I-2) to yield intermediate (I-3);
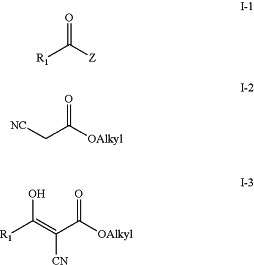 wherein
R1 is alkyl or aryl; and
Z is Cl or OAlkyl;
b) treating intermediate (I-3) with an alkylhalide (I-4) to yield intermediate (I-5);
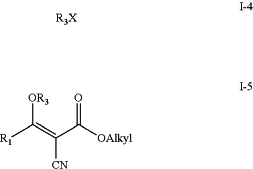 wherein
R3 is alkyl; and
X is halogen;
c) treating intermediate (I-5) with hydrazide (I-6) to yield a 5-amino pyrazole template intermediate (I-7);
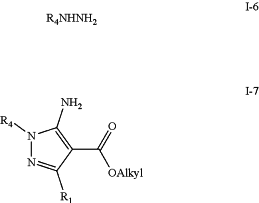 wherein
R4 is alkyl;
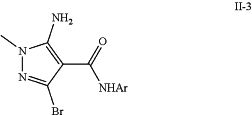
d) brominating and treating intermediate (I-7) with ArNH2 to effect an ester/amide exchange to yield a bromo-pyrazole intermediate (II-3);
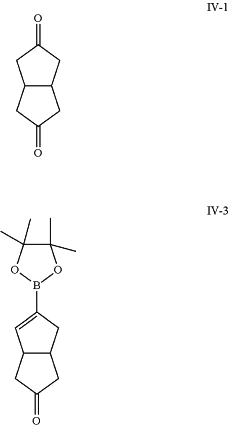
 e) selectively modifying diketone (IV-1) to yield boronate ester (IV-3);
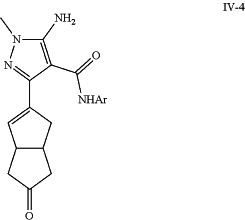
 f) coupling bromo-pyrazole intermediate (II-3) with boronate ester (IV-3) to yield intermediate (IV-4);
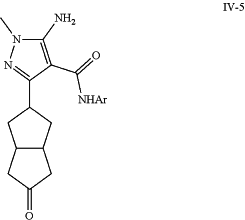
 g) reducing intermediate (IV-4) to yield intermediate (IV-5);
 and
either
h) converting ketone intermediate (IV-5) to epoxide (VI-1);
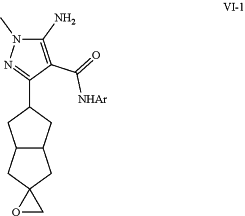 transforming epoxide (VI-1) to with a nucleophile RSH to form sulfide (VI-3);
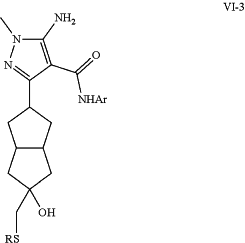 and
modifying sulfide (VI-3) to yield target sulfone (VI-5);
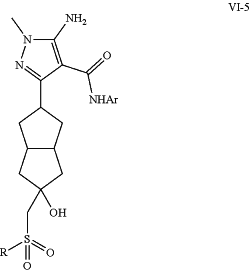 or
i) reacting ketone intermediate (IV-5) with a corresponding anion of sulfone, —SO2R, to yield target sulfone (VI-5)
 |