| CPC C01F 5/14 (2013.01) [B01D 53/62 (2013.01); C04B 9/20 (2013.01); C25B 1/20 (2013.01); B01D 2253/1124 (2013.01)] | 17 Claims |
|
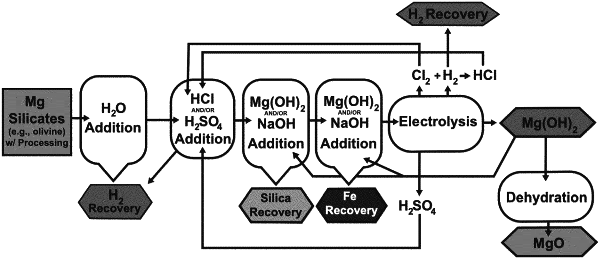
1. A method of processing a magnesium silicate source by the steps of:
selecting a magnesium silicate source selected from: olivine, serpentine, pyroxene, amphiboles, phyllosilicates, clays, and combinations thereof,
subjecting the selected magnesium silicate source to water digestion at a temperature of less than 120° C. and wherein the ratio, by mass, of magnesium silicate source to water in the water digestion is 1 part magnesium silicate to 1 to 20 parts water to form a washed magnesium silicate;
subjecting the washed magnesium silicate to acid digestion, to produce a digested solution, acid digestion comprising mixing the washed magnesium silicate at a temperature of less than 120° C. with hydrochloric acid or sulfuric acid sufficient to decrease the pH of the mixture to −1-6 and, recovering evolved hydrogen gas during acid digestion;
completing a base wash by increasing the digested solution pH by addition of an alkali solution at a temperature of less than 120° C., to produce a magnesium salt solution consisting of magnesium chloride, magnesium sulphate, or both magnesium chloride and magnesium sulphate, wherein the base wash comprises two pH increasing steps including:
a first increase in digested solution pH by at least 1 to 3 pH greater than the digested solution pH and removing precipitated silica from the magnesium salt solution; and
a subsequent pH increase in digested solution pH to a pH of 6.0 or higher and removing precipitated iron oxide from the magnesium salt solution; subjecting the magnesium salt solution to electrolysis at a temperature of less than 120° C.; and
recovering magnesium hydroxide produced from electrolysis from an electrolysis cathode and oxygen and chlorine from an electrolysis anode.
|