| CPC A61M 16/0003 (2014.02) [A61M 16/0051 (2013.01); A61M 16/024 (2017.08); A61M 16/06 (2013.01); A61M 16/085 (2014.02); A61M 16/0875 (2013.01); A61M 16/12 (2013.01); A61M 16/208 (2013.01); A61M 2016/0033 (2013.01); A61M 2016/0036 (2013.01); A61M 16/0078 (2013.01); A61M 16/0833 (2014.02); A61M 2016/1025 (2013.01); A61M 2016/1035 (2013.01); A61M 16/122 (2014.02); A61M 2202/0208 (2013.01); A61M 2202/0225 (2013.01); A61M 2202/025 (2013.01); A61M 2202/0275 (2013.01); A61M 2205/3327 (2013.01); A61M 2206/11 (2013.01); A61M 2210/0618 (2013.01)] | 15 Claims |
|
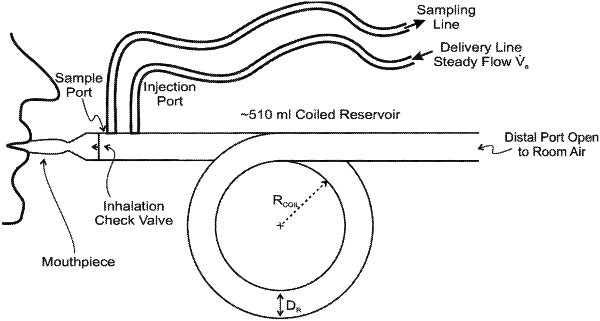
1. A device configured to administer at least one therapeutic gas comprising nitric oxide to a patient, comprising:
a. at least one reservoir tube, having a proximal and a distal end, wherein the distal end is configured to be open to the ambient environment such that gas moves through the distal end unimpeded and wherein the at least one reservoir tube has a volume larger than a tidal volume of the patient breath;
b. at least one therapeutic gas inlet at the proximal end of the at least one reservoir tube, wherein a delivery tube is connected to the at least one therapeutic gas inlet and at least one therapeutic gas source; and
c. a patient interface fluidly connected to the proximal end of the at least one reservoir tube via an inhalation check valve; wherein the patient interface is configured to form a gas-tight seal between the patient and the device; and
d. a sampling port, wherein the sampling port is distal to the inhalation check valve and proximal to the at least one therapeutic gas inlet.
|