| CPC A61L 24/06 (2013.01) [A61K 47/02 (2013.01); A61K 47/34 (2013.01); A61K 49/04 (2013.01); A61K 49/0409 (2013.01); A61L 24/0021 (2013.01); A61L 24/02 (2013.01); A61L 24/043 (2013.01); A61L 24/046 (2013.01); C08L 83/04 (2013.01); A61L 2400/06 (2013.01); A61L 2430/36 (2013.01)] | 18 Claims |
|
1. A method comprising: (a) forming a crosslinkable composition for vascular embolization comprising:
(i) providing a first fluid composition comprising (A) a crosslinkable polymer and (B) bismuth trioxide, wherein the bismuth trioxide has an average primary particle size from 80 nm to 200 nm;
(ii) providing a second fluid composition comprising (A) a crosslinker and (B) bismuth trioxide, wherein the bismuth trioxide has an average primary particle size from 80 nm to 200 nm; and
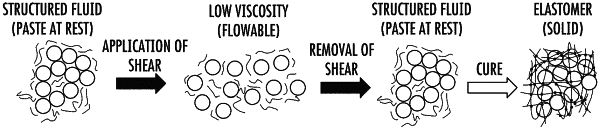
(iii) mixing the first fluid composition and the second fluid composition with a dry composition, the dry composition comprising a first silica filler and, optionally, a second silica filler that is the same or different from the first silica filler, wherein the first silica filler and the second silica filler, if present, are hydrophobic;
thereby forming the crosslinkable composition, wherein the bismuth trioxide in the first fluid composition, the bismuth trioxide in the second fluid composition, the first silica filler, and the optional second silica filler are substantially evenly dispersed in the crosslinkable composition;
(b) preparing the crosslinkable composition for injection; and
(c) injecting the crosslinkable composition into an injection site within a vasculature of a patient whereupon the crosslinkable composition substantially flows into and occludes a plurality of distal vessels in the vasculature, wherein at least one distal vessel in the plurality of distal vessels has a diameter of less than 100 microns, and whereafter the crosslinkable composition substantially flows into and occludes the plurality of distal vessels in the vasculature, the crosslinkable composition crosslinks and forms into a solid.
|