| CPC A61K 31/197 (2013.01) [A61K 9/0053 (2013.01); A61K 31/198 (2013.01); A61K 45/06 (2013.01); A61P 25/18 (2018.01)] | 25 Claims |
|
1. A method of treating epilepsy, NMDAR encephalitis, Parkinson's disease, cognitive deficits in Parkinson's disease, Alzheimer's disease, mild cognitive impairment, amyotrophic lateral sclerosis (ALS), Huntington's disease, bipolar disorder, bipolar mania, bipolar depression, treatment-refractory depression, cognitive deficits in depression, major depressive disorder, generalized anxiety disorder, major depressive disorder with mixed features, and cognition deficits associated with Huntington's disease, subjective cognitive decline, traumatic brain injury, or Lewy Body Dementia, the method comprising administering to a subject in need thereof an effective amount of
Compound 100:
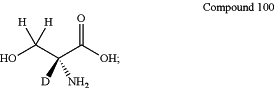 wherein each position designated specifically as deuterium has at least 90% incorporation of deuterium; and wherein the compound is at least about 90% stereomerically pure;
or a pharmaceutically acceptable salt thereof.
|