| CPC A61F 2/30942 (2013.01) [A61F 2/442 (2013.01); A61F 2/4611 (2013.01); A61F 2002/30065 (2013.01); A61F 2002/30952 (2013.01); A61F 2002/30971 (2013.01); A61F 2002/30985 (2013.01)] | 13 Claims |
|
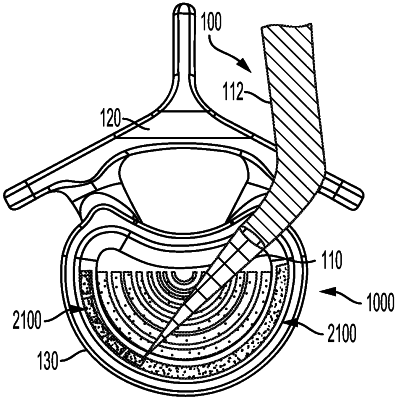
1. A motion-sparing spinal implant formed in-situ, within a patient, comprising:
a first endcap formed of a rigid material and having a size corresponding to a first vertebral body;
a second endcap formed of a rigid material and having a size corresponding to a second vertebral body; and
a pliable material configured to fill an interior space between the first and second endcaps, the pliable material being dispensed by a dispensing component and cured in-situ within the disc space of a patient;
wherein the first endcap and second endcap are formed of rigid material dispensed by the dispensing component.
|