| CPC A61B 5/4848 (2013.01) [A61B 5/0002 (2013.01); A61B 5/0031 (2013.01); A61B 5/24 (2021.01); A61B 5/377 (2021.01); A61B 5/0022 (2013.01); A61B 5/14539 (2013.01); A61B 5/14553 (2013.01); A61B 5/4082 (2013.01); A61B 5/4094 (2013.01)] | 17 Claims |
|
17. A method of assessing an effectiveness of an existing drug regimen to which a patient with an implanted medical device is subjected, the method comprising:
delivering, by the implanted medical device, electrical stimulation therapy to the patient's brain according to a set of stimulation parameters;
continuously sensing, by the implanted medical device, electrical activity of the patient's brain;
detecting, by the implanted medical device, epileptiform events in an electrographic signal corresponding to the sensed electrical activity;
detecting, by the implanted medical device, evoked responses in the electrographic signal, and calculating, in response to a detection of an evoked response, a measure of the evoked response;
detecting, by the implanted medical device, a presence of a specified frequency band in the electrographic signal, and calculating, in response to a detection of the specified frequency, a power measure of the electrographic signal in the specified frequency;
logging, by the implanted medical device, information as a function of time, the logged information comprising one or more of a count of occurrences of the epileptiform events, durations of the epileptiform events, measures of the evoked responses, and power measures of the electrographic signal in the specified frequency;
determining, by the implanted medical device, a tracked metric based on changes in the logged information over a time period within which the existing drug regimen, if effective, would affect the tracked metric;
determining, by the implanted medical device, the existing drug regimen is not effective responsive to the tracked metric not satisfying a criterion relative to a corresponding baseline metric; and
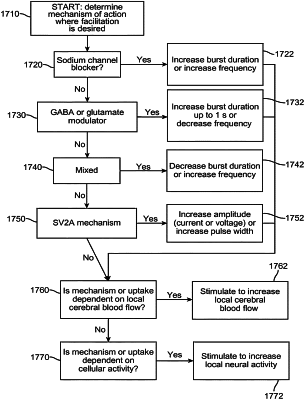
responsive to determining that the existing drug regimen is not effective, adjusting, by the implanted medical device, the set of stimulation parameters based on a mechanism of action of a drug of the existing drug regimen.
|