| CPC C07D 471/22 (2013.01) [A61P 31/18 (2018.01)] | 52 Claims |
|
1. A compound of Formula I:
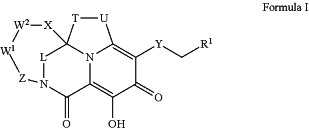 or a pharmaceutically acceptable salt thereof, wherein
R1 is H, C6-10aryl or C6-10heteroaryl, wherein the C6-10aryl or C6-10heteroaryl are optionally substituted with one to four RA1, wherein each RA1 is independently halo, C1-6alkyl, C1-4haloalkyl, C3-6cycloalkyl, cyano, —O—C1-4alkyl, —O—C3-6cycloalkyl, or C1-4alkyl-O—C1-4alkyl;
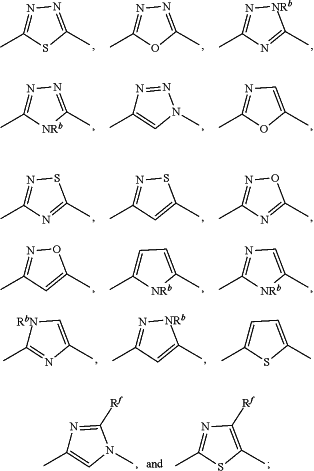
Y is selected from the group consisting of —C(O)NH—,
 L is —CR3aR3b—, —C(O)—, —SO2—, —CR3aR3b—CR3cR3d—, or —N(Ra)—;
W1 is a bond or —CR4aR4b—;
W2 is —CR5aR5b—, —CR5aR5bCR5cR5d—, —CR6a═CR6b—, —N(R7)—, —O—, —S(O)n—, —C(O)NRe—, —CR5aR5b—N(R7)—, —CR5aR5b—O—, —CR5aR5b—S(O)n—, —CR5aR5b—C(O)NRe—, —CR5aR5b—NRe—C(O)—, —S(O)nN(Re)—CR5aR5b—, or —N(Re)—S(O)n—CR5aR5b—;
X is a bond or —CR8aR8b—;
Z is —CR9aR9b—, —CR9aR9bCR9cR9d—, or —CR10a═CR10b—;
T is CR2aR2b— or —CR2aR2b—CR2cR2d—; U is —NR11—, —CR12aR12b—, —S(O)n—, —C(O)—, or —O—; or T and U together are
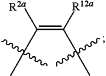 R2a, R2b, R2c, R2d, R12a, and R12b are independently H, C1-6alkyl, C1-6haloalkyl, C3-6cycloalkyl, C3-6halocycloalkyl, halo, cyano, —CH2Ra, —CH2ORa, —CH2—S(O)nRa, —ORa, —O—C(O)—NHRa, —NHRa, —C(O)—NH(Ra), —NRe—C(O)Ra, —NRe—S(O)nRa, —S(O)n—NH(Ra), or —S(O)n—Ra;
R3a, R3b, R3c, and R3d are independently H, C1-6alkyl, C1-4haloalkyl, or —O—C1-4alkyl;
R4a, R4b, R5a, R5b, R5c, R5d, R8a, R8b, R9a, R9b, R9c, and R9d are independently H, C1-6alkyl, C1-4haloalkyl, C3-6cycloalkyl, C3-6halocycloalkyl, halo, hydroxyl, cyano, —O—C1-4alkyl, or C1-4alkylene-O—C1-4alkyl;
each R6a, R6b, R10a, and R10b is independently H, halo, C1-4haloalkyl, or C1-6alkyl;
R7 is H, C1-6alkyl, C1-4haloalkyl, C3-6cycloalkyl, C3-6halocycloalkyl, C(O)Rc, or SO2Rc;
R11 is H, C1-4alkyl, C1-4haloalkyl, C3-6cycloalkyl, C3-6halocycloalkyl, —C(O)—Ra, —S(O)n—Ra, —CH2—Ra;
each Ra is independently (i) H, (ii) C1-6alkyl, (iii) C3-6cycloalkyl, (iv) a 5 to 10 membered carbocyclic ring, (v) 5 to 10 membered heterocyclic ring containing 1 or 2 heteroatom selected from N, O, and S, (vi) a 6 to 10 membered aromatic ring, or (iv) a 5 to 10 membered heteroaromatic ring containing 1 to 3 heteroatoms selected from N, O and S;
wherein the C1-6alkyl, C3-6cycloalkyl, 5 to 10 membered carbocyclic ring, 5 to 10 membered heterocyclic ring, 6 to 10 membered aromatic ring or 5 to 10 membered heteroaromatic ring of Ra is optionally substituted with 0 to 4 substituents independently selected from the group consisting of (i) oxo (ii) halo, (iii) cyano, (iv) —O—C1-4alkyl, (v) C1-6alkyl, (vi) —ORe (vii) 3 to 10 membered carbocyclic ring, (viii) 5 to 10 membered heterocyclic ring containing 1 or 2 heteroatom selected from N, O, and S, (ix) 6 to 10 membered aromatic ring, or (x) a 5 to 10 membered heteroaromatic ring containing 1 to 3 heteroatoms selected from N, O and S; wherein the 3 to 10 membered carbocyclic ring, 5 to 10 membered heterocyclic ring, 6 to 10 membered aromatic ring, or 5 to 10 membered heteroaromatic ring is optionally substituted with one to four RA5, wherein each RA5 is independently oxo, halo, cyano, C1-6alkyl, C3-6cycloalkyl or —ORe;
Rb is H, C1-4alkyl, C1-4haloalkyl or C3-6cycloalkyl;
Rf is H, halo, C1-4alkyl, or C1-4haloalkyl;
each Rc is independently, H, C1-4alkyl, C1-4haloalkyl, C3-6cycloalkyl, C3-6halocycloalkyl; C(O)Rd, or —SO2Rd;
each Rd is independently C1-4alkyl, C1-4haloalkyl, C3-6cycloalkyl, C3-6halocycloalkyl, —NRe2, or —ORe;
each Re is independently H, C1-4alkyl or C3-6cycloalkyl wherein each C1-4alkyl or C3-6cycloalkyl is optionally substituted by a halo or a cyano; and
each n is 0, 1, or 2.
|