| CPC C01G 31/02 (2013.01) | 21 Claims |
|
1. A method of recovering vanadium values from a shale deposit, comprising:
recovering an oxidized material from the shale deposit, the oxidized material comprising carbonate gangue, vanadiferous mineralization and oxidized vanadate-containing kerogen, wherein the oxidized material comprises:
≤1 wt % organic carbon,
≤2 wt % vanadium pentoxide, as an original oxidized vanadium fraction, and
≥10 wt % calcium and magnesium carbonates, as an original oxidized carbonates fraction;
recovering a carbonaceous material from the shale deposit comprising carbonate gangue and a vanadium-containing kerogen, wherein the carbonaceous material comprises:
≥4 wt % organic carbon,
≤2 wt % vanadium pentoxide, as an original carbonaceous vanadium fraction, and
≥10 wt % calcium and magnesium carbonates, as an original carbonaceous carbonates fraction;
subjecting the oxidized material to an attrition step and to a particle size separation step so as to segregate a vanadium fines stream from a coarser carbonates stream, wherein the vanadium fines stream comprises less than about 30% wt % of the original oxidized carbonates fraction and at least 75% wt % of the original oxidized vanadium fraction reports to the vanadium fines stream;
subjecting the carbonaceous material to a de-sliming step so as to segregate a fine vanadium and carbonate-containing fraction from a coarser vanadium and carbonate-containing fraction; and, subjecting the coarser vanadium-containing fraction to flotation so as to segregate a vanadium froth concentrate from a carbonate flotation tail, wherein the vanadium froth concentrate comprises less than about 30 wt % of the original carbonaceous carbonates fraction and at least 75 wt % of the original carbonaceous vanadium fraction reports to the combination of the vanadium froth concentrate and the fine vanadium and carbonate-containing fraction;
combining the vanadium froth concentrate, the fine vanadium and carbonate-containing fraction and the vanadium fines to provide a vanadium concentrate having a vanadium concentration of at least 1 wt %;
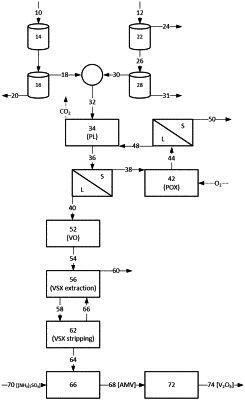
subjecting the vanadium concentrate to an atmospheric pre-leach (PL) with the addition of an autoclave-sourced sulfuric acid, to produce a pre-leached product stream, so as to:
decompose residual carbonate minerals in the vanadium concentrate to produce carbon dioxide off-gas; and,
precipitate iron species from the vanadium concentrate;
subjecting the pre-leached product to a PL solid liquid separation to separate a PL solid stream from a vanadium recovery solution;
directing the PL solid stream to a pressure oxidation (POX) in the presence of a POX leaching acid and a POX oxidizing agent, to produce a POX product stream by leaching vanadium into a POX solution comprising the autoclave-sourced sulfuric acid;
subjecting the POX product stream to a POX solid liquid separation to separate a solid POX residue from the POX solution;
directing the POX solution to the atmospheric PL, to provide the autoclave-sourced sulfuric acid to the atmospheric PL;
subjecting the vanadium recovery solution to a vanadium oxidation step to convert dissolved vanadium species to dissolved vanadium(v) oxoanion species in an oxidized vanadium recovery solution;
subjecting the oxidized vanadium recovery solution to a vanadium solvent extraction (VSX) step to load vanadium species into a pregnant organic phase, and separating the pregnant organic phase from a spent vanadium recovery solution;
stripping vanadium from the pregnant organic phase into an aqueous sodium or ammonium vanadate solution, to provide a stripped organic phase that is recycled to the VSX step; and,
precipitating ammonium metavanadate from the sodium or ammonium vanadate solution by addition of ammonium sulfate.
|