| US 11,696,906 B2 | ||
| Stable oral suspensions of baclofen | ||
| Sushant Omprakash Dube, Navi Mumbai (IN); Purushottam Sakhahari Pattewar, Hyderabad (IN); Pankaj Kisan Chatki, Hyderabad (IN); and Sumitra Ashokkumar Pillai, Hyderabad (IN) | ||
| Assigned to SLAYBACK PHARMA LLC, Princeton, NJ (US) | ||
| Filed by SLAYBACK PHARMA LLC, Princeton, NJ (US) | ||
| Filed on Dec. 22, 2022, as Appl. No. 18/087,625. | ||
| Application 18/087,625 is a continuation of application No. 17/948,882, filed on Sep. 20, 2022, granted, now 11,564,898, issued on Jan. 31, 2023. | ||
| Application 17/948,882 is a continuation of application No. 17/591,438, filed on Feb. 2, 2022, granted, now 11,484,518, issued on Nov. 1, 2022. | ||
| Claims priority of application No. 202141018869 (IN), filed on Apr. 23, 2021. | ||
| Prior Publication US 2023/0139109 A1, May 4, 2023 | ||
| This patent is subject to a terminal disclaimer. | ||
| Int. Cl. A61K 31/197 (2006.01); A61K 9/00 (2006.01); A61K 9/10 (2006.01) | ||
| CPC A61K 31/197 (2013.01) [A61K 9/10 (2013.01); A61K 9/0053 (2013.01)] | 8 Claims |
|
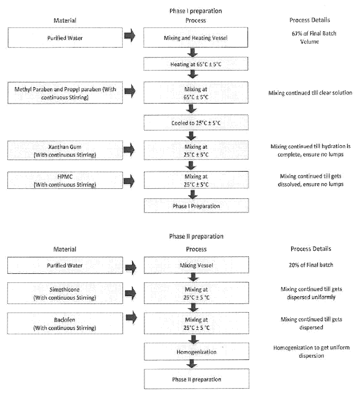
1. A stable liquid suspension comprising:
a. about 5 mg/mL of baclofen; b. at least one stabilizer; and c. at least one pharmaceutically acceptable liquid vehicle; wherein the suspension is in the form of a ready-to-use liquid suspension or a ready-to-administer liquid suspension that is suitable for oral administration to a subject in need thereof, and wherein no crystal growth is observed when suspension was stored for 3 months at 40° C./75% RH.
|