| CPC A61B 18/06 (2013.01) [A61M 5/14236 (2013.01); A61B 2017/00323 (2013.01); A61B 2018/0019 (2013.01); A61B 2018/00065 (2013.01); A61B 2018/00202 (2013.01); A61B 2018/00404 (2013.01); A61B 2218/002 (2013.01); A61M 2025/1086 (2013.01)] | 20 Claims |
|
1. A vein ablation system, comprising:
a catheter having an elongated body;
an ablation device at a distal portion of the elongated body; and
a control device at a proximal portion of the elongated body, the control device comprising:
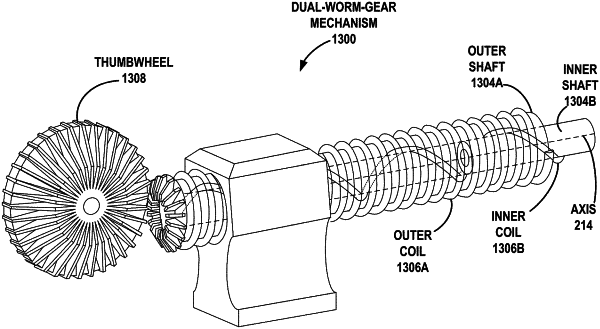
an input mechanism configured to simultaneously control:
an infusion of a chemical agent into a target vessel;
a longitudinal translation of the ablation device through the target vessel;
and
a dual coaxial worm gear comprising a first worm gear configured to drive the longitudinal translation and a second worm gear configured to infuse the chemical agent.
|