| CPC A23L 33/155 (2016.08) [A23L 29/10 (2016.08); A23L 29/219 (2016.08); A23L 29/25 (2016.08); A23L 33/125 (2016.08); A23L 33/15 (2016.08); A23P 10/28 (2016.08); A61K 8/671 (2013.01); A61K 9/2018 (2013.01); A61K 47/36 (2013.01); A23V 2002/00 (2013.01)] | 4 Claims |
|
1. A compressed tablet consisting of:
(a) about 24.48 wt. %, based on total weight of the compressed table, of solid particles;
(b) about 30.14 wt. %, based on total weight of the compressed tablet, of microcrystalline cellulose,
(c) about 45.20 wt. %, based on total weight of the compressed tablet, of calcium phosphate, and
(d) about 0.18 wt. %, based on total weight of the compressed tablet, of magnesium stearate, wherein
the solid particles consist of, based on the total weight of the solid particles, of:
(i) 27.00 wt. % of vitamin A acetate,
(ii) 0.75 wt. % of dl-α-tocopherol;
(iii) 0.75 wt. % of butylated hydroxytoluene (BHT);
(iii) 47.31 wt. % of food modified starch;
(iv) 18.19 wt. % of trehalose as a non-reducing sugar;
(v) 4.00 wt. % of corn starch; and
(vi) 2.00 wt. % of water, wherein
the compressed tablet is a compression product of compressing the components (a)-(d) at a pressure between 5-40 kN, and wherein
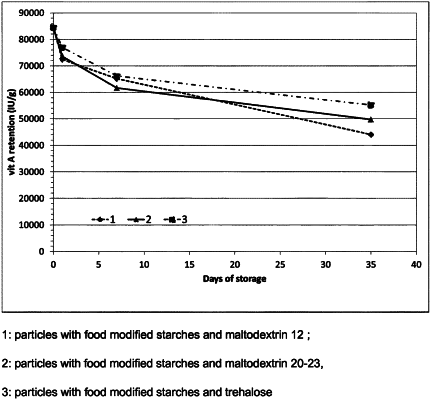
the compressed tablet has greater retention of the vitamin A acetate as compared to a similar compressed tablet that does not contain the trehalose after storage in a closed brown-glass bottle at room temperature for 35 days.
|