| CPC H02J 50/80 (2016.02) [A61M 60/875 (2021.01); H02J 50/10 (2016.02); H02J 50/90 (2016.02); A61M 2205/50 (2013.01); A61M 2205/58 (2013.01); H02J 2310/23 (2020.01)] | 18 Claims |
|
1. A system, comprising:
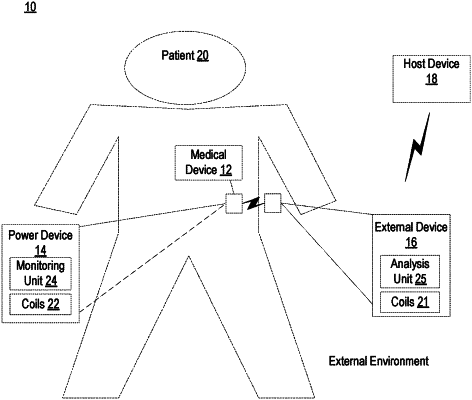
a power device implantable within a patient for powering an implantable medical device, the power device including:
a first coil configured to receive wireless power signals for powering the implantable medical device; and
processing circuitry configured to:
determine at least one measurable electrical characteristic in a plurality of electrical pathways in the power device including an electrical pathway to the first coil; and
detect reduced performance in receiving wireless power signals based at least in part on the at least one measurable electrical characteristic;
an external device positioned outside of the patient for providing power to the implantable medical device, the external device including:
a second coil for transmitting the wireless power signals; and
processing circuitry configured to:
receive an indication of power received by the power device; and
determine a wireless power transfer efficiency over a predetermined period of time based at least in part on the indication of the power received by the power device, wherein the wireless power transfer efficiency over the predetermined period of time corresponds to a long term moving average.
|