| CPC C07K 5/0827 (2013.01) [C07K 5/02 (2013.01); C07K 5/06086 (2013.01); C07K 5/08 (2013.01); C07K 5/0806 (2013.01); C07K 5/0808 (2013.01); C07K 5/081 (2013.01); C07K 5/0812 (2013.01); C07K 5/0815 (2013.01); C07K 5/0821 (2013.01); A61K 38/00 (2013.01)] | 5 Claims |
|
1. A process for the manufacture of a compound according to formula I:
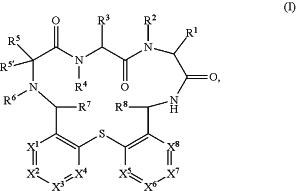 or a pharmaceutically acceptable salt thereof, wherein:
X1 is C-L1-R11 or N;
X2 is C-L2-R12 or N;
X3 is C-L3-R13 or N;
X4 is C-L4-R14 or N, with the proviso that not more than three of X1, X2, X3 and X4 are N;
X5 is C-L5-R15 or N;
X6 is C-L6-R16 or N;
X7 is C-L7 R17 or N;
X8 is C-L8-R18 or N, with the proviso that not more than three of X5, X6, X7 and X8 are N;
R1 is —(CH2)m-heteroaryl or —(CH2)m-heterocycloalkyl, wherein heteroaryl is optionally substituted with one or more halo, cyano, C1-7-alkyl, C1-7-haloalkyl, C3-7-cycloalkyl or C1-7-alkoxy;
R2, R4 and R6 are each individually selected from the group consisting of hydrogen, C1-7-alkyl, C1-7-haloalkyl, and C3-7-cycloalkyl;
R3 is —C1-7-alkyl, —(CH2)n—NR20R21, —(CH2)n—C(O)NR20R21 or —(CH2)n—O—(CH2)q—NR20R21;
R5 is hydrogen, C1-7-alkyl, hydroxy-C1-7-alkyl, —(CH2)o—NR22R23, —(CH2)o—C(O)—NR22R23, —(CH2)o—O—(CH2)q—NR20R21, —(CH2)o—NH—C(NH)—NR22R23, —(CH2)o—NH—C(O)—NR22R23, —(CH2)o—NH—C(O)—OR26, —(CH2)o—C3-7-cycloalkyl, —(CH2)o-heterocycloalkyl, —(CH2)o-heteroaryl, or —(CH2)o-aryl, wherein cycloalkyl, heterocycloalkyl, heteroaryl and aryl are optionally substituted by halo, cyano, C1-7-alkyl, C1-7-haloalkyl, C1-7-alkoxy or aryl;
R5′ is hydrogen;
R7 and R8 are each individually selected from the group consisting of hydrogen, C1-7-alkyl, C1-7-haloalkyl, C3-7-cycloalkyl and C1-7-alkoxy;
R11, R12, R13, R14, R15 and R16 are each individually selected from the group consisting of hydrogen, halogen, cyano, C1-7-alkyl, C1-7-haloalkyl, —NR24R25, C1-7-alkyl-NR24R25, hydroxy, C1-7-alkoxy, haloC1-7-alkoxy, —B(OH)2, benzyloxy-propynyl (—C≡C—CH2—O-benzyl), C3-7-cycloalkyl, heterocycloalkyl, aryl and heteroaryl, wherein heteroaryl is optionally substituted with one C1-7-haloalkyl or C1-7-alkoxy;
R17 is hydrogen, halogen, cyano, C1-7-alkyl, C1-7-haloalkyl, —NR24R25, C1-7-alkyl-NR24R25, hydroxy, C1-7-alkoxy, haloC1-7-alkoxy, B(OH)2, benzyloxy-prop-1-ynyl, C3-7-cycloalkyl, heterocycloalkyl, aryl or heteroaryl,
wherein heterocycloalkyl is optionally substituted with one —NR24R25,
wherein aryl and heteroaryl are optionally substituted with one, two or three substituents selected from the list group consisting of halogen, cyano, C1-7-alkyl, C1-7-haloalkyl, C1-7-hydroxyalkyl, hydroxy, C1-7-alkoxy, —NR24R25, —SO2—C1-7-alkyl, —SO2—NR24R25, heterocycloalkyl and heterocycloalkyl substituted with C1-7-alkyl;
R18 is hydrogen, halogen, cyano, C1-7-alkyl, C1-7-haloalkyl, hydroxy, C1-7-hydroxyalkyl, C1-7-alkoxy, C1-7-haloalkoxy, —NR24R25, C1-7-alkyl-NR24R25, C3-7-cycloalkyl, heterocycloalkyl, aryl or heteroaryl,
wherein aryl and heteroaryl are optionally substituted with one, two or three substituents selected from the group consisting of halogen, cyano, C1-7-alkyl C1-7-haloalkyl, hydroxy, C1-7-alkoxy, —NR24R25, C1-7-alkyl-NR24R25, —CO—NH—(CH2)r—NR24R25, —CO—NH—(CH2)r—OH, —CO—NH—(CH2)r-heterocycloalkyl, —CO—OH, —O—C1-7-hydroxyalkyl, —O—(CH2)r—CO—OH, —SO2—C1-7-alkyl, —SO2—NR24R25, heterocycloalkyl, —O-heterocycloalkyl and heterocycloalkyl substituted with C1-7-alkyl;
R20 and R22 are each individually selected from the group consisting of hydrogen, C1-7-alkyl, C1-7-haloalkyl, C3-7-cycloalkyl and —C(═NH)—NH2;
R21 and R23 are each individually selected from the group consisting of hydrogen and C1-7-alkyl;
R24 and R25 are each individually selected from the group consisting of hydrogen, C1-7-alkyl, C1-7-haloalkyl, C1-7-hydroxyalkyl and C3-7-cycloalkyl;
R26 is hydrogen, C1-7-alkyl or benzyl;
L1, L2, L3, L4, L5, L6, L7 and L8 are each individually selected from the group consisting of a single bond, —C(O)—, —SO2—, —(CH2)p—, —CH═CH— and —C≡C—;
m is 1, 2, 3, or 4;
n is 1, 2, 3, or 4;
o is 0, 1, 2, 3, or 4;
p is 1, 2, 3, or 4;
q is 1, 2, 3, or 4; and
r is 1, 2, 3, or 4;
the process comprising the steps of:
a) reacting a compound of formula (III) with a compound of formula (IV) using sodium cyanoborohydride (NaCNBH3) to provide a compound of formula (II);
 b) cleaving off the protecting group (PG) and the resin from the compound of formula (II); and
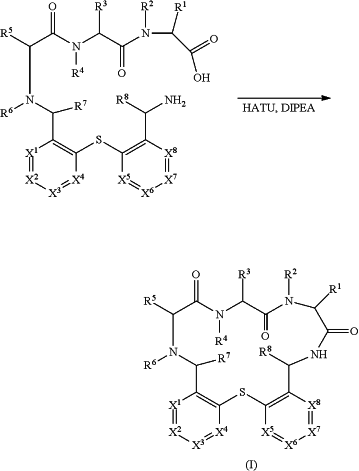 c) followed by cyclisation of the cleaved compound of formula (II) using HATU and Hünig's base.
|