| CPC C07D 495/04 (2013.01) [A61K 45/06 (2013.01); A61K 47/06 (2013.01); A61P 31/12 (2018.01)] | 12 Claims |
|
1. A compound of formula (I), or a pharmaceutically acceptable salt thereof:
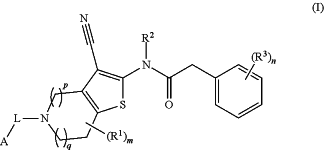 wherein:
A is phenyl or 3-6 membered cycloalkyl, wherein the 3-6 membered cycloalkyl is optionally substituted with —C1-6alkyl, cyano, —C1-4aminoalkyl, —C1-4alkoxyl, —C1-6haloalkyl, —C1-4haloalkoxyl, or halogen; and wherein the phenyl is substituted with —C1-6alkyl, cyano, —C1-4aminoalkyl, —C1-4alkoxyl, —C1-6haloalkyl, —C1-4haloalkoxyl, or halogen;
L is —C1-6alkylene-;
each R1 is independently selected from —C1-6alkyl, cyano, —C1-4aminoalkyl, —C1-4alkoxyl, —C1-6haloalkyl, —C1-4haloalkoxyl, and halogen;
R2 is H or —C1-6alkyl;
each R3 is independently selected from —C1-6alkyl, —CN, —C1-4alkoxyl, —C1-6haloalkyl, —C1-4 haloalkoxyl, halogen, —C(O)R3a, —C(O)OR3b, —C(O)NR3cR3d, —P(O)R3eR3f, —P(O)(OR3g)(OR3h), —P(O)(OR3i)(R3j), —S(O)2R3k, —S(O)2NR3lR3m, —S(O)R3n, —NR3oR3p, —NR3qC(O)R3r, —N(R3s)C(O)OR3t and —NR3uS(O)2R3v, wherein each of the —C1-6alkyl, —C1-4alkoxyl, —C1-6haloalkyl, and —C1-4 haloalkoxyl is independently optionally substituted by hydroxyl, —NR3wR3x, —C1-4alkoxyl, —S(O)2NR3yR3z, or —S(O)2R3a2; wherein each of R3w, R3x, R3y, and R3z is independently H, —C1-4alkyl or —C1-6haloalkyl and R3a2 is —C1-4alkyl or —C1-6haloalkyl; or
any two R3 may combine with one atom to form a 5-6 membered fused heterocycloalkyl, wherein the heterocycloalkyl comprises one or two heteroatoms selected from N and S, and wherein the heterocycloalkyl is independently optionally substituted with one or two groups selected from —C1-6alkyl, —C1-4aminoalkyl —CN, —C1-4alkoxyl, halogen, —C1-6haloalkyl and —C1-4haloalkoxyl;
each of R3a, R3b, R3c, R3d, R3e, Rf, R3g, R3h, R3i, R3j, R3k, R3n, R3o, R3p, R3q, R3r, R3s, R3t, R3u, R3v is independently selected from H, —C1-6alkyl and —C1-6haloalkyl;
each of R3l and R3m is independently selected from H, —C1-6alkyl, —C1-6haloalkyl, -aminoalkyl, and -hydroxyalkyl, wherein the —C1-6alkyl is optionally further substituted by 3-6 membered cycloalkyl, and wherein the 3-6 membered cycloalkyl substituent is optionally further substituted by 1 or 2 halogens;
p is 1;
q is 1;
m is 0, 1 or 2; and
n is 0, 1 or 2.
|