| CPC C07D 487/04 (2013.01) [C07D 209/14 (2013.01); C07D 401/06 (2013.01); C07D 401/08 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/06 (2013.01); C07D 403/08 (2013.01); C07D 403/10 (2013.01); C07D 403/12 (2013.01); C07D 405/06 (2013.01); C07D 405/12 (2013.01); C07D 409/06 (2013.01); C07D 409/08 (2013.01); C07D 409/12 (2013.01); C07D 413/06 (2013.01); C07D 413/08 (2013.01); C07D 413/12 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 495/04 (2013.01); C07D 498/04 (2013.01); C07D 513/04 (2013.01)] | 18 Claims |
|
1. A method of treatment of Dengue virus or yellow fever virus, by the administration of an effective amount of a compound of formula B:
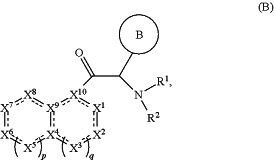 or a pharmaceutically acceptable salt thereof, optionally in combination with one or more other medicines, to a human patient in need thereof,
wherein
the moiety
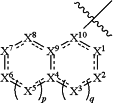 is
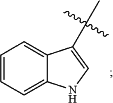 wherein the wavy line (
 ) indicates the point of attachment to the carbonyl of the main formula (B); wherein said moiety is substituted with one or more Z1; ) indicates the point of attachment to the carbonyl of the main formula (B); wherein said moiety is substituted with one or more Z1;cycle B is
 wherein the wavy line (
 ) indicates the point of attachment to the carbon atom of the main formula (B), and wherein the depicted cycles are substituted with one, two, or three Z1a; ) indicates the point of attachment to the carbon atom of the main formula (B), and wherein the depicted cycles are substituted with one, two, or three Z1a;R1 is phenyl; wherein said phenyl is substituted with one, two, or three Z1b;
R2 is hydrogen;
each Z1 and Z1a is independently selected from the group consisting of halogen, hydroxyl, —OZ2, —O—C(═O)Z3, ═O, —S(═O)2Z3, —S(═O)2NZ4Z5, trifluoromethyl, trifluoromethoxy, —NZ4Z5, —NZ4C(═O)Z2, —NZ4C(═O)OZ2, cyano, —C(═O)Z3, —C(═O)OZ2, —C(═O)NZ4Z5, C1-6alkyl, heteroC1-6alkyl, aryl, heterocycle, and heterocycle-C1-6alkyl;
and wherein said C1-6alkyl, heteroC1-6alkyl, aryl, heterocycle, and heterocycle-C1-6alkyl, are optionally substituted with one, two, or three substituents selected from hydroxyl, ═O, halogen, trifluoromethyl, —OCF3, —O—C(O)Me, cyano, nitro, —C(O)OH, —C(O)OC1-6alkyl, —NH2, —NHCH3; —N(CH3)2, —NH—C(═O)O—C1-4alkyl, —S(O)2C1-4alkyl, and —O—C1-6alkyl;
each Z1b is independently selected from the group consisting of hydroxyl, —OZ2, —O—C(═O)Z3, ═O, —S(═O)2Z3, —S(═O)2NZ4Z5, trifluoromethyl, trifluoromethoxy, —NZ4Z5, —NZ4C(═O)Z2, —NZ4C(═O)OZ2, cyano, —C(═O)Z3, —C(═O)OZ2, —C(═O)NZ4Z5, C3-6alkyl, heteroC1-6alkyl, aryl, heterocycle, and heterocycle-C1-6alkyl;
and wherein said C3-6alkyl, heteroC1-6alkyl, aryl, heterocycle, and heterocycle-C1-6alkyl, are optionally substituted with one, two, or three substituents selected from hydroxyl, ═O, halogen, trifluoromethyl, —OCF3, —O—C(O)Me, cyano, nitro, —C(O)OH, —C(O)OC1-6alkyl, —NH2, —NHCH3; —N(CH3)2, —NH—C(═O)O—C1-4alkyl, —S(O)2C1-4alkyl, and —O—C1-6alkyl;
each Z2 is independently selected from C1-6alkyl, aryl, heterocycle, and heterocycle-C1-6alkyl;
wherein said C1-6alkyl, aryl, heterocycle, and heterocycle-C1-6alkyl, are optionally substituted with one, two, or three substituents selected from hydroxyl, ═O, halogen, trifluoromethyl, difluoromethyl, —O—C1-6alkyl, —OCF3, —S(═O)2C1-4alkyl, cyano, —C(═O)OH, —C(═O)O—C1-4alkyl, —NH2, —N(CH3)2, pyrrolidinyl, piperidinyl, and piperazinyl, wherein said C1-6alkyl is not substituted with hydroxyl;
each Z3 is independently selected from hydroxyl, aryl, and heterocycle;
wherein said aryl and heterocycle are optionally substituted with one, two, or three substituents selected from C1-6alkyl and —N(CH3)2;
each Z4 and Z5 is independently selected from hydrogen, C1-6alkyl, aryl, C3-7cycloalkyl, and heterocycle;
or an isomer thereof, or a solvate thereof, or a salt thereof, or a prodrug thereof;
wherein the term “heterocycle” means a saturated, unsaturated, or aromatic ring system of 3 to 18 atoms including at least one N, O, S, or P.
|