| CPC C07D 401/14 (2013.01) [C07D 405/14 (2013.01); C07D 413/06 (2013.01); C07D 413/14 (2013.01); C07D 417/06 (2013.01); C07D 417/14 (2013.01)] | 39 Claims |
|
1. A method of treating activity-induced muscle damage, a neuromuscular condition, a metabolic myopathy, or a movement disorder,
wherein the neuromuscular condition is selected from Duchenne Muscular Dystrophy, Becker muscular dystrophy, myotonic dystrophy 1, myotonic dystrophy 2, facioscapulohumeral muscular dystrophy, oculopharyngeal muscular dystrophy, limb girdle muscular dystrophy, tendinitis, and carpal tunnel syndrome;
wherein the movement disorder comprises muscle spasticity associated with multiple sclerosis; Parkinson's disease; Alzheimer's disease; cerebral palsy; injury; or a traumatic event, wherein the traumatic event is selected from stroke, traumatic brain injury, spinal cord injury, hypoxia, meningitis, encephalitis, phenylketonuria, and amyotrophic lateral sclerosis;
wherein the metabolic myopathy is selected from McArdle's syndrome;
the method comprising administering to a subject in need thereof a compound or salt of Formula (III′):
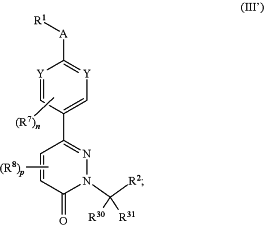 or a salt thereof, wherein:
each Y is independently selected from C(R3), N, and N+(—O−);
A is absent or selected from —O—, —NR4—, —CR5R6—, —C(O)—, —S—, —S(O)—, and —S(O)2—;
R1 is selected from:
C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, ═O, ═S, ═N(R10), —CN, C3-10 carbocycle, and 3- to 10-membered heterocycle, wherein the C3-10 carbocycle and 3- to 10-membered heterocycle are each optionally substituted with one or more R9; and
C3-10 carbocycle and 3- to 10-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, ═O, ═S, ═N(R10), and —CN; or
R1 together with R3 form a 5- to 10-membered heterocycle or C5-10 carbocycle, wherein the 5- to 10-membered heterocycle or C5-10 carbocycle is optionally substituted with one or more R9; or R1 together with R5 form a 3- to 10-membered heterocycle or saturated C3-10 carbocycle, wherein the 3- to 10-membered heterocycle or saturated C3-10 carbocycle is optionally substituted with one or more R9; or R1 together with R4 form a 3- to 10-membered heterocycle, wherein the 3- to 10-membered heterocycle is optionally substituted with one or more R9; and
when A is —NR4—, R1 is additionally selected from hydrogen, and when A is —C(O)—, R1 is additionally selected from —N(R10)2 and —OR10;
when A is absent, R1 is further selected from halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, and —CN;
R2 is a heteroaryl optionally substituted with one or more substituents independently selected from:
halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, ═O, ═S, ═N(R10), and —CN; and when R2 is pyridyl or pyrimidyl, a substituent on a nitrogen atom of the pyridyl or pyrimidyl is optionally further selected from —O—;
C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, ═O, ═S, ═N(R10), —CN, C3-10 carbocycle and 3- to 10-membered heterocycle, wherein the C3-10 carbocycle and 3- to 10-membered heterocycle are each optionally substituted with one or more R9; and
C3-10 carbocycle and 3- to 10-membered heterocycle, each of which is optionally substituted with one or more R9;
each R3, R5, and R6 are independently selected from:
hydrogen, halogen, —OR10, —SR10, —N(R10)2, —NO2, —CN, and C1-6 alkyl optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —NO2, and —CN; or
R3 together with R1 form a 5- to 10-membered heterocycle or C5-10 carbocycle, wherein the 5- to 10-membered heterocycle or C5-10 carbocycle is optionally substituted with one or more R9; or R5 together with R1 form a 3- to 10-membered heterocycle or C3-10 carbocycle, wherein the 3- to 10-membered heterocycle or C3-10 carbocycle is optionally substituted with one or more R9;
R4 is independently selected from:
hydrogen; and
C1-6 alkyl optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —NO2, and —CN; or R4 together with R1 form a 3- to 10-membered heterocycle, which is optionally substituted with one or more R9;
each R7 and R8 is independently selected from:
halogen, —OR10, —SR10, —N(R10)2, —NO2, —CN, and C1-6 alkyl optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —NO2, and —CN;
each R9 is independently selected from:
halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, ═O, ═S, ═N(R10), and —CN; and
C1-3 alkyl, C2-3 alkenyl, and C2-3 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, —OR10, —SR10, —N(R10)2, —C(O)R10, —C(O)N(R10)2, —N(R10)C(O)R10, —N(R10)C(O)N(R10)2, —OC(O)N(R10)2, —N(R10)C(O)OR10, —C(O)OR10, —OC(O)R10, —S(O)R10, —S(O)2R10, —NO2, ═O, ═S, ═N(R10), and —CN;
each R10 is independently selected from:
hydrogen; and
C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl, each of which is optionally substituted with one or more substituents independently selected from halogen, —CN, —OH, —SH, —NO2, —NH2, ═O, ═S, —O—C1-6 alkyl, —S—C1-6 alkyl, —N(C1-6 alkyl)2, —NH(C1-6 alkyl), C3-10 carbocycle, and 3- to 10-membered heterocycle; and
C3-10 carbocycle, and 3- to 10-membered heterocycle, each of which is optionally substituted with one or more substituents independently selected from halogen, —CN, —OH, —SH, —NO2, —NH2, ═O, ═S, —O—C1-6 alkyl, —S—C1-6 alkyl, —N(C1-6 alkyl)2, —NH(C1-6 alkyl), C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 carbocycle, 3- to 10-membered heterocycle, and haloalkyl;
R30 and R31 are independently selected from R10; or R30 and R31 come together to form a C3-7 carbocycle or 3- to 7-membered heterocycle, wherein C3-7 carbocycle or 3- to 7-membered heterocycle is optionally substituted with one or more R9;
n is 0, 1, or 2; and
p is 0, 1, or 2.
|