| CPC C07D 401/06 (2013.01) [A61P 29/00 (2018.01); C07D 231/18 (2013.01); C07D 233/84 (2013.01); C07D 249/04 (2013.01); C07D 249/12 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/06 (2013.01); C07D 405/04 (2013.01); C07D 405/06 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 413/06 (2013.01)] | 20 Claims |
|
1. A compound of formula (I):
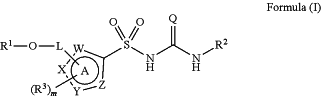 Formula (I)
or a pharmaceutically acceptable salt or solvate thereof, wherein:
Q is selected from O or S;
W, X, Y and Z are each independently N, O, S, NH or CH, wherein at least one of W, X, Y and Z is N or NH, such that ring A is a 5-membered heteroaryl group containing at least one nitrogen atom in the 5-membered ring structure;
L is a saturated or unsaturated hydrocarbylene group, wherein the hydrocarbylene group may be straight-chained or branched, or be or include cyclic groups, wherein the hydrocarbylene group may optionally be substituted, and wherein the hydrocarbylene group may optionally include one or more heteroatoms N, O or S in its carbon skeleton;
R1 is hydrogen or a saturated or unsaturated hydrocarbyl group, wherein the hydrocarbyl group may be straight-chained or branched, or be or include cyclic groups, wherein the hydrocarbyl group may optionally be substituted, and wherein the hydrocarbyl group may optionally include one or more heteroatoms N, O or S in its carbon skeleton;
optionally L and R1 together with the oxygen atom to which they are attached may form a 3- to 12-membered saturated or unsaturated cyclic group, wherein the cyclic group may optionally be substituted;
R2 is a cyclic group substituted at the α and α′ positions, wherein R2 may optionally be further substituted;
each R3 is independently a halo, —OH, —NO2, —NH2, —N3, —SH, —SO2H, —SO2NH2, or a saturated or unsaturated hydrocarbyl group, wherein the hydrocarbyl group may be straight-chained or branched, or be or include cyclic groups, wherein the hydrocarbyl group may optionally be substituted, and wherein the hydrocarbyl group may optionally include one or more heteroatoms N, O or S in its carbon skeleton;
m is 0, 1, 2 or 3; and
optionally any R3, and any two adjacent W, X, Y or Z, may together form a 3- to 12-membered saturated or unsaturated cyclic group fused to ring A, wherein the cyclic group fused to ring A may optionally be substituted;
provided that any atom of L or R1 that is directly attached to W, X, Y or Z is not the same atom of L or R1 that is directly attached to the oxygen atom of R1—O-L-, and that the oxygen atom of R1—O-L- is not directly attached to a sp2 hybridised carbon atom; and
wherein in an optionally substituted group or moiety:
(i) each hydrogen atom may optionally be replaced by a group independently selected from halo; —CN; —NO2; —N3; —Rβ; —OH; —ORβ; —Rα-halo; —Rα—CN; —Rα—NO2; —Rα—N3; —Rα—Rβ; —Rα—OH; —Rα—ORβ; —SH; —SRβ; —SORβ; —SO2H; —SO2Rβ; —SO2NH2; —SO2NHRβ; —SO2N(Rβ)2; —Rα—SH; —Rα—SRβ; —Rα—SORβ; —Rα—SO2H; —Rα—SO2Rβ; —Rα—SO2NH2; —Rα—SO2NHRβ; —Rα—SO2N(Rβ)2; —Si(Rβ)3; —O—Si(Rβ)3; —Rα—Si(Rβ)3; —Rα—O—Si(Rβ)3; —NH2; —NHRβ; —N(Rβ)2; —N(O)(Rβ)2; —N+(Rβ)3; —Rα—NH2; Rα—NHRβ; Rα—N(Rβ)2; —Rα—N(O)(Rβ)2; —Rα—N+(Rβ)3, —CHO; —CORβ; —COOH; —COORβ; —OCORβ; —Rα—CHO; —Rα—CORβ; —Rα—COOH; —Rα—COORβ; —Rα—OCORβ; —C(═NH)Rβ; —C(═NH)NH2; —C(═NH)NHRβ; —C(═NH)N(Rβ)2; —C(═NRβ)Rβ; —C(═NRβ)NHRβ; —C(═NRβ)N(Rβ)2; —C(═NOH)Rβ; —C(N2)Rβ; —Rα—C(═NH)Rβ; —Rα—C(═NH)NH2; —Rα—C(═NH)NHRβ; —Rα—C(═NH)N(Rβ)2; —Rα—C(═NRβ)Rβ; —Rα—C(═NRβ)NHRβ; —Rα—C(═NRβ)N(Rβ)2; —Rα—C(═NOH)Rβ; —Rα—C(N2)Rβ; —NH—CHO; —NRβ—CHO; —NH—CORβ; —NRβ—CORβ; —CONH2; —CONHRβ; —CON(Rβ)2; —Rα—NH—CHO; —Rα—NRβ—CHO; —Rα—NH—CORβ; —Rα—NRβ—CORβ; —Rα—CONH2; —Rα—CONHRβ; —Rα—CON(Rβ)2; —O—Rα—OH; —O—Rα—ORβ; —O—Rα—NH2; —O—Rα—NHRβ; —O—Rα—N(Rβ)2; —O—Rα—N(O)(Rβ)2; —O—Rα—N+(Rβ)3; —NH—Rα—OH; —NH—Rα—ORβ; —NH—Rα—NH2; —NH—Rα—NHRβ; —NH—Rα—N(Rβ)2; —NH—Rα—N(O)(Rβ)2; —NH—Rα—N+(Rβ)3; —NRβ—Rα—OH; —NRβ—Rα—ORβ; —NRβ—Rα—NH2; —NRβ—Rα—NHRβ; —NRβ—Rα—N(Rβ)2; —NRβ—Rα—N(O)(Rβ)2; —NRβ—Rα—N+(Rβ)3; —N(O)Rβ—Rα—OH; —N(O)Rβ—Rα—ORβ; —N(O)Rβ—Rα—NH2; —N(O)Rβ—Rα—NHRβ; —N(O)Rβ—Rα—N(Rβ)2; —N(O)Rβ—Rα—N(O)(Rβ)2; —N(O)Rβ—Rα—N+(Rβ)3; —N+(Rβ)2—Rα—OH; —N+(Rβ)2—Rα—ORβ; —N+(Rβ)2—Rα—NH2; —N+(Rβ)2—Rα—NHRβ; —N+(Rβ)2—Rα—N(Rβ)2; or —N+(Rβ)2—Rα—N(O)(Rβ)2; and/or
(ii) any two hydrogen atoms attached to the same atom may optionally be replaced by a π-bonded substituent independently selected from oxo (═O), ═S, ═NH or ═NRβ; and/or
(iii) any two hydrogen atoms attached to the same or different atoms, within the same optionally substituted group or moiety, may optionally be replaced by a bridging substituent independently selected from —O—, —S—, —NH—, —N═N—, —N(Rβ)—, —N(O)(Rβ)—, —N+(Rβ)2— or —Rα—;
wherein each —Rα— is independently selected from an alkylene, alkenylene or alkynylene group, wherein the alkylene, alkenylene or alkynylene group contains from 1 to 6 atoms in its backbone, wherein one or more carbon atoms in the backbone of the alkylene, alkenylene or alkynylene group may optionally be replaced by one or more heteroatoms N, O or S, wherein one or more —CH2— groups in the backbone of the alkylene, alkenylene or alkynylene group may optionally be replaced by one or more —N(O)(Rβ)— or —N+(Rβ)2— groups, and wherein the alkylene, alkenylene or alkynylene group may optionally be substituted with one or more halo and/or —Rβ groups; and
wherein each —Rβ is independently selected from a C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl or C2-C6 cyclic group, or wherein any two or three —Rβ attached to the same nitrogen atom may, together with the nitrogen atom to which they are attached, form a C2-C7 cyclic group, and wherein any —Rβ may optionally be substituted with one or more C1-C4 alkyl, C1-C4 haloalkyl, C3-C7 cycloalkyl, C3-C7 halocycloalkyl, —O(C1-C4 alkyl), —O(C1-C4 haloalkyl), —O(C3-C7 cycloalkyl), —O(C3-C7 halocycloalkyl), —CO(C1-C4 alkyl), —CO(C1-C4 haloalkyl), —COO(C1-C4 alkyl), —COO(C1-C4 haloalkyl), halo, —OH, —NH2, —CN, —C≡CH, oxo (═O), or 4- to 6-membered heterocyclic group.
|