| CPC C07C 69/63 (2013.01) [C07C 67/08 (2013.01); C07C 67/307 (2013.01); C07C 67/54 (2013.01); C07D 307/33 (2013.01)] | 20 Claims |
|
1. A process for preparing the 2-bromoglutaric acid diester of formula (I) below:
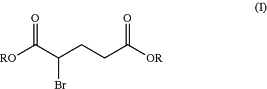 in which R represents a propyl, isopropyl, n-butyl, isobutyl, sec-butyl, pentyl or hexyl group,
wherein said process comprises the following steps:
(b) forming the 2-hydroxyglutaric acid diester of formula (II)
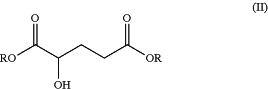 by reacting butyrolactone acid of formula (BA)
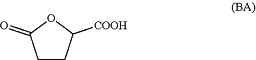 with the alcohol of formula ROH, in the presence of an acid;
(c) brominating the 2-hydroxyglutaric acid diester of formula (II), by sparging with gaseous hydrobromic acid, thereby leading to the 2-bromoglutaric acid diester of formula (I); and
wherein a degree of purity of the 2-bromoglutaric acid diester of formula (I), determined by HPLC analysis, is greater than or equal to 90%.
|