| CPC C07C 311/29 (2013.01) [C07C 69/16 (2013.01); C07C 309/42 (2013.01); C07C 311/51 (2013.01); C07C 317/22 (2013.01); C07D 205/06 (2013.01); C07D 205/12 (2013.01); C07D 207/404 (2013.01); C07D 211/54 (2013.01); C07D 213/71 (2013.01); C07D 233/84 (2013.01); C07D 235/16 (2013.01); C07D 239/38 (2013.01); C07D 249/08 (2013.01); C07D 249/10 (2013.01); C07D 263/32 (2013.01); C07D 277/30 (2013.01); C07D 295/26 (2013.01); C07D 305/08 (2013.01); C07D 307/64 (2013.01); C07D 487/10 (2013.01)] | 19 Claims |
|
1. A compound having structural formula:
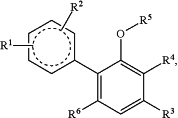 or an enantiomer, diastereomer, racemate, or tautomer thereof, or a pharmaceutically acceptable salt, solvate or hydrate of the compound, enantiomer, diastereomer, racemate, or tautomer, wherein
the
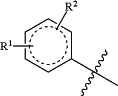 moiety is
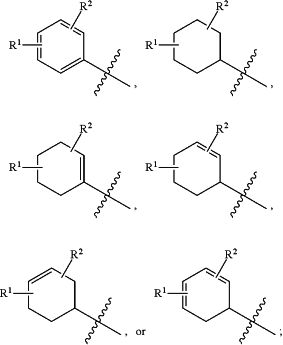 R1 is hydrogen, halo, C1-C8 alkyl, C2-C8 alkenyl, C2-C8 alkynyl, —CO2H, —(C0-C4 alkyl)-C(O)O(C1-C6 alkyl), —(C0-C4 alkyl)-OC(O)O—(C1-C6 alkyl), —OR1a, —(C1-C4 alkyl)OR1a, —SR1a, —(C1-C4 alkyl)SR1a, —NR1bR1c, —(C1-C4 alkyl)NR1bR1c, —(C1-C4 alkyl)C(O)NR1bR1c, —(C0-C4 alkyl)-aryl, —(C0-C4 alkyl)-heteroaryl, —(C0-C4 alkyl)-cycloalkyl, —(C0-C4 alkyl)-heterocycloalkyl, or oxo when attached to a ring of appropriate saturation, wherein
R1a, R1b, and R1c are independently hydrogen, C1-C4 alkyl, or —C(O)(C1-C4) alkyl;
R2 is hydrogen, halo, C1-C8 alkyl, C2-C8 alkenyl, C2-C8 alkynyl, —CO2H, —(C0-C4 alkyl)-C(O)O(C1-C6 alkyl), —(C0-C4 alkyl)-OC(O)O—(C1-C6 alkyl), —OR2a, —(C1-C4 alkyl)OR2a, —SR2a, —(C1-C4 alkyl)SR2a, —NR2bR2c, —(C1-C4 alkyl)NR2bR2c, —(C1-C4 alkyl)C(O)NR2bR2c, —(C0-C4 alkyl)-aryl, —(C0-C4 alkyl)-heteroaryl, —(C0-C4 alkyl)-cycloalkyl, —(C0-C4 alkyl)-heterocycloalkyl, or oxo when attached to a ring of appropriate saturation, wherein
R2a, R2b, and R2c are independently hydrogen, C1-C4 alkyl, or —C(O)(C1-C4) alkyl;
R3 is C1-C12 alkyl;
R4 is
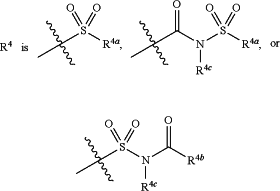 wherein
R4a is C1-C8 alkyl, C2-C8 alkenyl, C2-C8 alkynyl, —OR4c, —(C1-C4 alkyl)OR4c, —(C1-C4 alkyl)-C(O)(C1-C4 alkyl), —(C0-C4 alkyl)-aryl, —(C0-C4 alkyl)-heteroaryl, —(C0-C4 alkyl)-cycloalkyl, —(C0-C4 alkyl)-heterocycloalkyl, —NR4dR4e, or —(C1-C4 alkyl)NR4dR4e, and
R4b is C1-C8 alkyl, C2-C8 alkenyl, C2-C8 alkynyl, —CH2—(OCH2CH2)0-6O(C1-C8 alkyl), —(C1-C4 alkyl)OR4e, —(C1-C4 alkyl)-C(O)(C1-C4 alkyl), —(C1-C4 alkyl)-C(O)O(R4e), —(C0-C4 alkyl)-aryl, —(C0-C4 alkyl)-heteroaryl, —(C0-C4 alkyl)-cycloalkyl, —(C0-C4 alkyl)-heterocycloalkyl, or —(C1-C4 alkyl)NR4dR4e, wherein
R4d is hydrogen, C1-C6 alkyl, —(C1-C4 alkyl)-C(O)(C1-C4 alkyl), —(C1-C4 alkyl)-C(O)O(R4e), —(C1-C4 alkyl)-OC(O)(R4e), —(C0-C4 alkyl)-aryl, —(C0-C4 alkyl)-heteroaryl, —(C0-C4 alkyl)-cycloalkyl, or —(C0-C4 alkyl)-heterocycloalkyl,
R4e is hydrogen or C1-C4 alkyl, and
R4c is hydrogen or C1-C4 alkyl;
R5 is hydrogen, C1-C8 alkyl, —(C1-C4 alkyl)-O—(C0-C4 alkyl), —C(O)(C1-C4 alkyl), —C(O)O(C1-C4 alkyl), —C(O)NH2, —C(O)NH(C1-C4 alkyl), —C(O)N(C1-C4 alkyl)2, —(C1-C4 alkyl)C(O)O(C1-C4 alkyl), or —(C1-C4 alkyl)OC(O)(C1-C4 alkyl); and
R6 is hydrogen or —OR6a, wherein R6a is hydrogen, C1-C8 alkyl, —(C1-C4 alkyl)-O—(C0-C4 alkyl), —C(O)(C1-C4 alkyl), —C(O)O(C1-C4 alkyl), —C(O)NH2, —C(O)NH(C1-C4 alkyl), —C(O)N(C1-C4 alkyl)2, —(C1-C4 alkyl)C(O)O(C1-C4 alkyl), or —(C1-C4 alkyl)OC(O)(C1-C4 alkyl); wherein
each alkyl, alkenyl and alkynyl is unsubstituted, halogenated, substituted with one or two hydroxyl or C1-C6 alkoxy groups, or substituted with one or two oxo groups;
each cycloalkyl has 3-10 ring carbons and is saturated or partially unsaturated, and optionally includes one or two fused cycloalkyl rings, each fused ring having 3-8 ring members, and is substituted with 0-6 R7;
each heterocycloalkyl has 3-10 ring members and 1-3 heteroatoms where each is independently nitrogen, oxygen or sulfur and is saturated or partially unsaturated, and optionally includes one or two fused cycloalkyl rings, each having 3-8 ring members, and is substituted with 0-6 R7;
each aryl is a phenyl or a naphthyl, and optionally includes one or two fused cycloalkyl or heterocycloalkyl rings, each fused cycloalkyl or heterocycloalkyl ring having 4-8 ring members, and is substituted with 0-5 R8;
each heteroaryl is a 5-6 membered monocyclic heteroaryl ring having 1-4 heteroatoms, where each is independently nitrogen, oxygen or sulfur or a 8-10 membered bicyclic heteroaryl having 1-5 heteroatoms where each is independently nitrogen, oxygen or sulfur, and optionally includes one or two fused cycloalkyl or heterocycloalkyl rings, each fused cycloalkyl or heterocycloalkyl ring having 4-8 ring members, and is substituted with 0-5 R8,
in which
each R7 is independently oxo, C1-C4 alkyl, —Cl, —F, —Br, —CN, —SF5, —N3, nitro, —SRA, —S(O)1-2RA, —ORA, —(C0-C3 alkyl)—ORB, —NRBRA, —C(O)RA, —C(O)NRBRA, —NRBC(O)RA, —C(S)NRBRA, —NRBC(S)RA, —CO2RA, —OC(O)RA, —C(O)SRA, —SC(O)RA, —C(S)ORA, —OC(S)RA, —C(S)SRA, —SC(S)RA, —S(O)1-2ORA, —OS(O)1-2RA, —S(O)1-2NRBRA, —NRBS(O)1-2RA, —OCO2RA, —OC(O)NRBRA, —NRBCO2RA, —NRBC(O)NRBRA, —SCO2RA, —OC(O)SRA, —SC(O)SRA, —SC(O)NRBRA, —NRBC(O)SRA, —OC(S)ORA, —OC(S)NRBRA, —NRBC(S)ORA, —NRBC(S)NRBRA, —SC(S)ORA, —OC(S)SRA, —SC(S)SRA, —SC(S)NRBRA, —NRBC(S)SRA, —NRBC(NRB)NRBRA, —NRBS(O)1-2NRBRA, phenyl, C3-C8 cycloalkyl, a five to six-membered heteroaryl, or a five to six-membered heterocycloalkyl; and
each R8 is independently optionally-substituted C1-C4 alkyl, —Cl, —F, —Br, —CN, —SF5, —N3, nitro, —SRA, —S(O)1-2RA, —ORA, —(C0-C3 alkyl)-ORB, —NRBRA, —C(O)RA, —C(O)NRBRA, —NRBC(O)RA, —C(S)NRBRA, —NRBC(S)RA, —CO2RA, —OC(O)RA, —SO3H,—C(O)SRA, —SC(O)RA, —C(S)ORA, —OC(S)RA, —C(S)SRA, —SC(S)RA, —S(O)1-2ORA, —OS(O)1-2RA, —S(O)1-2NRBRA, —NRBS(O)1-2RA, —OCO2RA, —OC(O)NRBRA, —NRBCO2RA, —NRBC(O)NRBRA, —SCO2RA, —OC(O)SRA, —SC(O)SRA, —SC(O)NRBRA, —NRBC(O)SRA, —OC(S)ORA, —OC(S)NRBRA, —NRBC(S)ORA, —NRBC(S)NRBRA, —SC(S)ORA, —OC(S)SRA, —SC(S)SRA, —SC(S)NRBRA, —NRBC(S)SRA, —NRBC(NRB)NRBRA, —NRBS(O)1-2NRBRA; phenyl, C3-C8 cycloalkyl, a five to six-membered heteroaryl, or a five to six-membered heterocycloalkyl;
wherein each RA is independently H or C1-C3 alkyl, and each RB is independently H, C1-C3 alkyl, C1-C3 fluoroalkyl, C1-C3 hydroxyalkyl, —S(O)1-2(C1-C3 alkyl), —C(O)(C1-C3 alkyl) or —CO2(C1-C3 alkyl).
|