| CPC C07C 211/63 (2013.01) [C07C 209/68 (2013.01); C07C 209/84 (2013.01); C07B 2200/13 (2013.01)] | 20 Claims |
|
1. A cage silicate consisting of anion component 1 represented by following formula (1), anion component 2 which is a mineral acid ion, cation component 1 represented by following formula (2), and cation component 2 which is an alkali ion, wherein
a ratio of the mole number of water to the mole number in terms of SiO2, (H2O/SiO2), is 0.7 to 30,
a ratio of the mole number of alkali ions to the mole number in terms of SiO2, (alkali ions/SiO2), is 1.0×10−7 to 1.0×10−2, and
a ratio of the mole number of the mineral acid ions to the mole number in terms of SiO2, (mineral acid ions/SiO2), is 1.0×10−7 to 1.0×10−3:
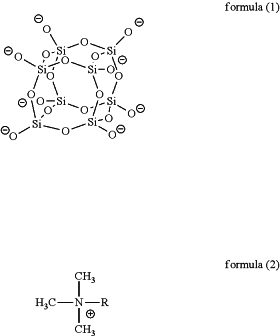 wherein in formula (2), R represents an alkyl group having 2 to 20 carbon atoms.
|